当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of N-(benzyloxy)-1,3-diphenyl-1H-pyrazole-4-carboxamide derivatives as potential antiproliferative agents by inhibiting MEK
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2016-09-04 08:23:01 Xian-Hai Lv, Zi-Li Ren, Ben-Guo Zhou, Qing-Shan Li, Ming-Jie Chu, Dao-Hong Liu, Kai Mo, Li-Song Zhang, Xiao-Kang Yao, Hai-Qun Cao
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2016-09-04 08:23:01 Xian-Hai Lv, Zi-Li Ren, Ben-Guo Zhou, Qing-Shan Li, Ming-Jie Chu, Dao-Hong Liu, Kai Mo, Li-Song Zhang, Xiao-Kang Yao, Hai-Qun Cao
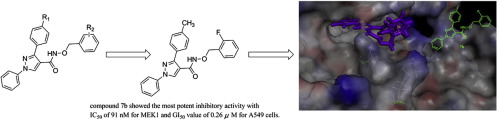
|
Mitogen activated protein kinase (MAPK) signal transduction pathway has been proved to play an important role in tumorigenesis and cancer development. MEK inhibitor has been demonstrated significant clinical benefit for blocking MAPK pathway activation and possibly could block reactivation of the MAPK pathway at the time of BRAF inhibitor resistance. Twenty N-(benzyloxy)-1,3-diphenyl-1H-pyrazole-4-carboxamide derivatives have been designed and synthesized as MEK inhibitors, and their biological activities were evaluated. Among these compounds, compound 7b showed the most potent inhibitory activity with IC50 of 91nM for MEK1 and GI50 value of 0.26μM for A549 cells. The SAR analysis and docking simulation were performed to provide crucial pharmacophore clues that could be used in further structure optimization.
中文翻译:

通过抑制MEK发现N-(苄氧基)-1,3-二苯基-1H-吡唑-4-羧酰胺衍生物作为潜在的抗增殖剂
丝裂原活化蛋白激酶(MAPK)信号转导途径已被证明在肿瘤发生和癌症发展中起重要作用。已经证明MEK抑制剂对于阻断MAPK途径活化具有显着的临床益处,并且可能在BRAF抑制剂抵抗时阻断MAPK途径的再活化。设计并合成了二十种N-(苄氧基)-1,3-二苯基-1H-吡唑-4-羧酰胺衍生物作为MEK抑制剂,并对其生物学活性进行了评估。在这些化合物中,化合物7b对MEK1和GI 50表现出最强的抑制活性,IC 50为91nM。A549细胞的0.26μM值。进行了SAR分析和对接模拟,以提供可用于进一步结构优化的关键药效团线索。
更新日期:2016-09-05
中文翻译:

通过抑制MEK发现N-(苄氧基)-1,3-二苯基-1H-吡唑-4-羧酰胺衍生物作为潜在的抗增殖剂
丝裂原活化蛋白激酶(MAPK)信号转导途径已被证明在肿瘤发生和癌症发展中起重要作用。已经证明MEK抑制剂对于阻断MAPK途径活化具有显着的临床益处,并且可能在BRAF抑制剂抵抗时阻断MAPK途径的再活化。设计并合成了二十种N-(苄氧基)-1,3-二苯基-1H-吡唑-4-羧酰胺衍生物作为MEK抑制剂,并对其生物学活性进行了评估。在这些化合物中,化合物7b对MEK1和GI 50表现出最强的抑制活性,IC 50为91nM。A549细胞的0.26μM值。进行了SAR分析和对接模拟,以提供可用于进一步结构优化的关键药效团线索。






























 京公网安备 11010802027423号
京公网安备 11010802027423号