Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2022-06-21 , DOI: 10.1016/j.molstruc.2022.133551
D. Rajaraman , L. Athishu Anthony , G. Sundararajan , M. Shanmugam , A. Arunkumar
|
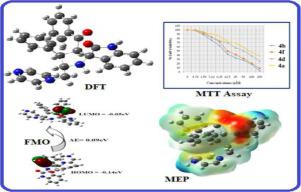
A series of seven novel spirooxindoles derivatives were synthesized by a three-component 1,3-dipolar cycloaddition method. The reaction proceeds through the formation of azomethine ylides generated in situ by the decarboxylative condensation of isatin and amine with dipolarophile chalcones. The antioxidant results revealed that the IC50 value of compounds 4a, 4b, 4d and 4f are very excellent against hydroxyl radical (IC50 = 15.9 to 30.3 µg/ml) and superoxide radical scavenging activity (IC50 = 14.7 to 22.2 µg/ml) whereas standard antioxidant Vitamin C showed their IC50 value only in the range of 25.0 and 20.0 µg/ml. Cytotoxic activity by MTT assay revealed that the compounds 4a, 4b, 4d and 4f exhibited broad inhibition on the KB cell lines with IC50 values of 6.5, 9.5, 32.5 and 55 µM, respectively. The observed IC50 values revealed that compound 4b possessed more inhibitory effect against the cancer cells. The anticancer activities of these derivatives were studied using molecular docking studies and it is compared with their experimental results. In addition, good oral bioavailability was predicted for all compounds by in silico calculations of ADME (absorption, distribution, metabolism, and elimination) and pharmacokinetic parameters. Finally the geometrical structure was optimized by density functional theory (DFT) method at B3LYP/6–31 G (d, p) as the basic set. The HOMO-LUMO energies describe the charge transfer takes place within the molecule. Molecular electrostatic potential has been analyzed.

































 京公网安备 11010802027423号
京公网安备 11010802027423号