当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of gem-Difluoroalkenes via Ni-Catalyzed Three-Component Defluorinative Reductive Cross-Coupling of Organohalides, Alkenes and Trifluoromethyl Alkenes
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2022-06-18 , DOI: 10.1002/cjoc.202200277 Teng Ma 1 , Xiao Li 1 , Yuanyuan Ping 1 , Wangqing Kong 1
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2022-06-18 , DOI: 10.1002/cjoc.202200277 Teng Ma 1 , Xiao Li 1 , Yuanyuan Ping 1 , Wangqing Kong 1
Affiliation
|
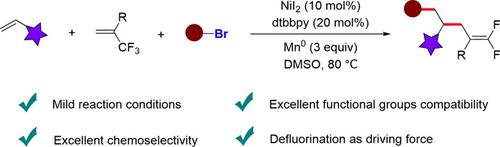
gem-Difluoroalkenes are considered ideal isosteres for metabolically susceptible carbonyl groups in modern drug discovery and medicinal chemistry. In addition, gem-difluoroalkenes are used as versatile precursors for the synthesis of difluoroalkylated compounds and monofluoroalkenes. Therefore, a great deal of effort has been devoted to developing efficient methods for their preparation. The catalytic defluorinative functionalization of trifluoromethyl alkenes represents a useful strategy for the preparation of chiral gem-difluoroalkenes. However, most of these catalytic processes are still essentially limited to two-component defluorinative cross-couplings to form single C—C bonds. Due to the challenge of controlling chemoselectivity in the carbon-carbon bond forming events, three-component defluorinative cross-coupling involving multiple C—C bond formations has rarely been studied. We report a nickel-catalyzed three-component defluorinative reductive cross-coupling of organohalides, alkenes and trifluoromethyl alkenes. A variety of electron-rich and electron-deficient alkenes, as well as aryl and alkyl halides can efficiently participate in the formation of three-component cross-coupling products. This reaction proceeds under mild conditions and exhibits excellent functional group compatibility without requiring a pendant chelating group, providing a variety of functionalized gem-difluoroalkenes in good yields with excellent chemoselectivity.
中文翻译:

Ni催化有机卤化物、烯烃和三氟甲基烯烃三组分脱氟还原交叉偶联合成偕二氟烯烃
gem -二氟烯烃被认为是现代药物发现和药物化学中易受代谢影响的羰基的理想等排体。此外,偕二氟烯烃用作合成二氟烷基化化合物和单氟烯烃的通用前体。因此,人们致力于开发有效的制备方法。三氟甲基烯烃的催化脱氟官能化代表了制备手性宝石的有用策略-二氟烯烃。然而,这些催化过程中的大多数仍然基本上限于双组分脱氟交叉偶联以形成单个CC键。由于在碳-碳键形成事件中控制化学选择性的挑战,涉及多个碳-碳键形成的三组分脱氟交叉偶联很少被研究。我们报告了有机卤化物、烯烃和三氟甲基烯烃的镍催化三组分脱氟还原交叉偶联。多种富电子和缺电子烯烃,以及芳基和烷基卤化物可以有效地参与三组分交叉偶联产物的形成。该反应在温和条件下进行,并表现出优异的官能团相容性,无需侧基螯合基团,gem -二氟烯烃具有良好的收率和优异的化学选择性。
更新日期:2022-06-18

中文翻译:

Ni催化有机卤化物、烯烃和三氟甲基烯烃三组分脱氟还原交叉偶联合成偕二氟烯烃
gem -二氟烯烃被认为是现代药物发现和药物化学中易受代谢影响的羰基的理想等排体。此外,偕二氟烯烃用作合成二氟烷基化化合物和单氟烯烃的通用前体。因此,人们致力于开发有效的制备方法。三氟甲基烯烃的催化脱氟官能化代表了制备手性宝石的有用策略-二氟烯烃。然而,这些催化过程中的大多数仍然基本上限于双组分脱氟交叉偶联以形成单个CC键。由于在碳-碳键形成事件中控制化学选择性的挑战,涉及多个碳-碳键形成的三组分脱氟交叉偶联很少被研究。我们报告了有机卤化物、烯烃和三氟甲基烯烃的镍催化三组分脱氟还原交叉偶联。多种富电子和缺电子烯烃,以及芳基和烷基卤化物可以有效地参与三组分交叉偶联产物的形成。该反应在温和条件下进行,并表现出优异的官能团相容性,无需侧基螯合基团,gem -二氟烯烃具有良好的收率和优异的化学选择性。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号