当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New (arylalkyl)azole derivatives showing anticonvulsant effects could have VGSC and/or GABAAR affinity according to molecular modeling studies
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2016-09-04 07:34:52
Suat Sari, Arzu Karakurt, Harun Uslu, F. Betül Kaynak, Ünsal Çalış, Sevim Dalkara
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2016-09-04 07:34:52
Suat Sari, Arzu Karakurt, Harun Uslu, F. Betül Kaynak, Ünsal Çalış, Sevim Dalkara
|
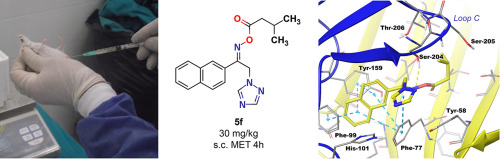
(Arylalkyl)azoles (AAAs) emerged as a novel class of antiepileptic agents with the invention of nafimidone and denzimol. Several AAA derivatives with potent anticonvulsant activities have been reported so far, however neurotoxicity was usually an issue. We prepared a set of ester derivatives of 1-(2-naphthyl)-2-(1H-1,2,4-triazol-1-yl)ethanone oxime and evaluated their anticonvulsant and neurotoxic effects in mice. Most of our compounds were protective against maximal electroshock (MES)- and/or subcutaneous metrazol (s.c. MET)-induced seizures whereas none of them showed neurotoxicity. Nafimidone and denzimol have an activity profile similar to that of phenytoin or carbamazepine, both of which are known to inhibit voltage-gated sodium channels (VGSCs) as well as to enhance γ-aminobutiric acid (GABA)-mediated response. In order to get insights into the effects of our compounds on VGSCs and A-type GABA receptors (GABAARs) we performed docking studies using homology model of Na+ channel inner pore and GABAAR as docking scaffolds. We found that our compounds bind VGSCs in similar ways as phenytoin, carbamazepine, and lamotrigine. They showed strong affinity to benzodiazepine (BZD) binding site and their binding interactions were mainly complied with the experimental data and the reported BZD binding model.
中文翻译:

根据分子模型研究,显示出新的抗惊厥作用的(芳烷基)唑衍生物可能具有VGSC和/或GABAAR亲和力
(芳烷基)唑(AAAs)出现了新的一类抗癫痫药,它是萘啶酮和地唑的发明。到目前为止,已经报道了几种具有有效的抗惊厥活性的AAA衍生物,但是神经毒性通常是一个问题。我们制备了一组1-(2-萘基)-2-(1H-1,2,4-三唑-1-基)乙酮肟的酯衍生物,并评估了它们在小鼠中的抗惊厥作用和神经毒性作用。我们的大多数化合物对最大电击(MES)和/或皮下注射甲硝唑(sc MET)引起的癫痫发作均具有保护作用,而它们均未显示神经毒性。萘啶酮和地唑酚具有与苯妥英钠或卡马西平类似的活性,已知两者均抑制电压门控钠通道(VGSC)并增强γ-氨基丁酸(GABA)介导的反应。甲RS)我们进行对接使用Na的同源性模型的研究+通道内的孔和GABA甲R作为对接支架。我们发现我们的化合物以与苯妥英钠,卡马西平和拉莫三嗪相似的方式结合VGSC。它们显示出对苯并二氮杂(BZD)结合位点的强亲和力,并且它们的结合相互作用主要与实验数据和报道的BZD结合模型相符。
更新日期:2016-09-04
中文翻译:

根据分子模型研究,显示出新的抗惊厥作用的(芳烷基)唑衍生物可能具有VGSC和/或GABAAR亲和力
(芳烷基)唑(AAAs)出现了新的一类抗癫痫药,它是萘啶酮和地唑的发明。到目前为止,已经报道了几种具有有效的抗惊厥活性的AAA衍生物,但是神经毒性通常是一个问题。我们制备了一组1-(2-萘基)-2-(1H-1,2,4-三唑-1-基)乙酮肟的酯衍生物,并评估了它们在小鼠中的抗惊厥作用和神经毒性作用。我们的大多数化合物对最大电击(MES)和/或皮下注射甲硝唑(sc MET)引起的癫痫发作均具有保护作用,而它们均未显示神经毒性。萘啶酮和地唑酚具有与苯妥英钠或卡马西平类似的活性,已知两者均抑制电压门控钠通道(VGSC)并增强γ-氨基丁酸(GABA)介导的反应。甲RS)我们进行对接使用Na的同源性模型的研究+通道内的孔和GABA甲R作为对接支架。我们发现我们的化合物以与苯妥英钠,卡马西平和拉莫三嗪相似的方式结合VGSC。它们显示出对苯并二氮杂(BZD)结合位点的强亲和力,并且它们的结合相互作用主要与实验数据和报道的BZD结合模型相符。

































 京公网安备 11010802027423号
京公网安备 11010802027423号