当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of solvents on the excited-state intramolecular proton transfer in 3-HTC
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2022-06-16 , DOI: 10.1002/poc.4402
Yao‐Dong Song 1 , Qian‐Ting Wang 2, 3, 4, 5, 6 , Wei‐wei Gao 1 , Zhixiong He 1 , Yan Wu 1
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2022-06-16 , DOI: 10.1002/poc.4402
Yao‐Dong Song 1 , Qian‐Ting Wang 2, 3, 4, 5, 6 , Wei‐wei Gao 1 , Zhixiong He 1 , Yan Wu 1
Affiliation
|
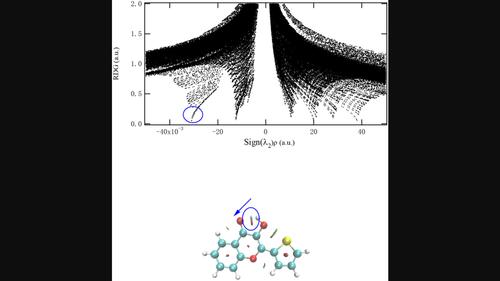
It has been demonstrated experimentally that proton transfer can occur in the excited state of 3-hydroxy-2-mercapto-4-one (3-HTC) molecule. However, the effect of solvent polarity on excited-state intramolecular proton transfer of 3-HTC has not been reported. In this paper, the molecular structures of ground and excited states in different solvents are optimized by density functional theory and time-dependent density functional theory. Based on the optimized structure, infrared vibration frequency, electronic spectra, natural bond orbital (NBO) population, and potential energy curves were calculated and analyzed. By analyzing the bond lengths and the bond angles related to the hydrogen bond, it can be seen that the intramolecular hydrogen bond intensity of 3-HTC molecule in the S1 state is strengthened in different solvents. With the increase of the solvent polarity, the degree of hydrogen bond strength decreases. Through the analysis of the infrared vibration frequency, it could be found that intramolecular hydrogen bond of 3-HTC is weakened as the solvent polarity increases. By analyzing the frontier molecular orbitals and the charge density difference (CDD) maps, it is proved that charge transfer occurs during photoexcitation, which promotes the excited-state proton transfer process. Further, the analysis of potential energy curves of 3-HTC in the ground state and S1 state shows that proton transfer can be realized in the S1 state of 3-HTC in different solvents. With the increase of solvent polarity, the difficulty of intramolecular proton transfer is increasing.
中文翻译:

溶剂对 3-HTC 激发态分子内质子转移的影响
实验证明,质子转移可以在 3-hydroxy-2-mercapto-4-one (3-HTC) 分子的激发状态下发生。然而,溶剂极性对 3-HTC 激发态分子内质子转移的影响尚未见报道。本文通过密度泛函理论和时变密度泛函理论对不同溶剂中基态和激发态的分子结构进行了优化。基于优化后的结构,计算分析了红外振动频率、电子光谱、自然键轨道(NBO)布居和势能曲线。通过分析与氢键相关的键长和键角,可以看出S 1中3-HTC分子的分子内氢键强度状态在不同溶剂中得到加强。随着溶剂极性的增加,氢键强度降低。通过红外振动频率分析可以发现,随着溶剂极性的增加,3-HTC分子内的氢键减弱。通过分析前沿分子轨道和电荷密度差(CDD)图,证明光激发过程中发生电荷转移,促进了激发态质子转移过程。此外,对3-HTC在基态和S 1态的势能曲线分析表明,在S 1中可以实现质子转移。3-HTC 在不同溶剂中的状态。随着溶剂极性的增加,分子内质子转移的难度越来越大。
更新日期:2022-06-16
中文翻译:

溶剂对 3-HTC 激发态分子内质子转移的影响
实验证明,质子转移可以在 3-hydroxy-2-mercapto-4-one (3-HTC) 分子的激发状态下发生。然而,溶剂极性对 3-HTC 激发态分子内质子转移的影响尚未见报道。本文通过密度泛函理论和时变密度泛函理论对不同溶剂中基态和激发态的分子结构进行了优化。基于优化后的结构,计算分析了红外振动频率、电子光谱、自然键轨道(NBO)布居和势能曲线。通过分析与氢键相关的键长和键角,可以看出S 1中3-HTC分子的分子内氢键强度状态在不同溶剂中得到加强。随着溶剂极性的增加,氢键强度降低。通过红外振动频率分析可以发现,随着溶剂极性的增加,3-HTC分子内的氢键减弱。通过分析前沿分子轨道和电荷密度差(CDD)图,证明光激发过程中发生电荷转移,促进了激发态质子转移过程。此外,对3-HTC在基态和S 1态的势能曲线分析表明,在S 1中可以实现质子转移。3-HTC 在不同溶剂中的状态。随着溶剂极性的增加,分子内质子转移的难度越来越大。

































 京公网安备 11010802027423号
京公网安备 11010802027423号