Synlett ( IF 1.7 ) Pub Date : 2022-06-15 , DOI: 10.1055/a-1854-2131 José Augusto Berrocal 1 , James Robert Hemmer 1
|
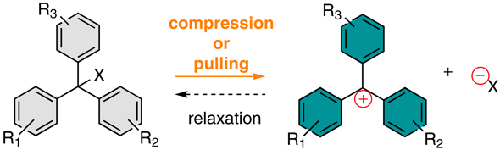
Triarylmethane derivatives and their corresponding trityl carbocations are among the oldest chemical species synthesized and studied by chemists. The carbocationic platforms are particularly interesting due to their stability, high extinction coefficient, and tunable absorption of light in the visible spectrum, which can be achieved through structural modifications. These stable cations are traditionally obtained through heterolytic cleavage of judiciously designed, parent triarylmethanes by exposure to acids or UV light (λ < 300 nm), and methods based on electrochemistry or radiolysis. Our group has recently discovered that trityl carbocations can be generated also via mechanical stimulation of solid polymer materials featuring triarylmethane units as covalent crosslinks. In this Synpacts contribution, we expand on our previous finding by discussing some intriguing research questions that we aim to tackle in the immediate future.
1 Introduction
2 The Development of Our First Triarylmethane Mechanophore
3 The Potential Reversibility of Triarylmethane Mechanophores
4 A General Molecular Platform for Force-Induced, Scissile, Homolytic and Heterolytic Bond Cleavage?
5 Conclusion
中文翻译:

三芳基甲烷机械载体中力诱导的杂解键断裂的观点
三芳基甲烷衍生物及其相应的三苯甲基碳正离子是化学家合成和研究的最古老的化学物种之一。碳阳离子平台因其稳定性、高消光系数和对可见光谱中的光的可调谐吸收而特别有趣,这可以通过结构修改来实现。这些稳定的阳离子传统上是通过暴露于酸或紫外光(λ < 300 nm)以及基于电化学或辐射分解的方法对精心设计的母体三芳基甲烷进行异解裂解获得的。我们小组最近发现,三苯甲基碳正离子也可以通过机械刺激以三芳基甲烷单元为共价交联的固体聚合物材料产生。在这个 Synpacts 贡献中,
1 简介
2 我们的第一个三芳基甲烷机械载体的开发
3 三芳基甲烷机械载体的潜在可逆性
4 力诱导、易断裂、均裂和杂裂键断裂的通用分子平台?
5 结论


















































 京公网安备 11010802027423号
京公网安备 11010802027423号