当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Trifluoroacetic Acid-Mediated Denitrogenative ortho-Hydroxylation of 1,2,3-Benzotriazin-4(3H)-ones: A Metal-Free Approach
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-06-14 , DOI: 10.1021/acs.joc.2c00354 Kanagaraj Madasamy 1 , Madasamy Hari Balakrishnan 2 , Ramaraju Korivi 1 , Subramaniyan Mannathan 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-06-14 , DOI: 10.1021/acs.joc.2c00354 Kanagaraj Madasamy 1 , Madasamy Hari Balakrishnan 2 , Ramaraju Korivi 1 , Subramaniyan Mannathan 1
Affiliation
|
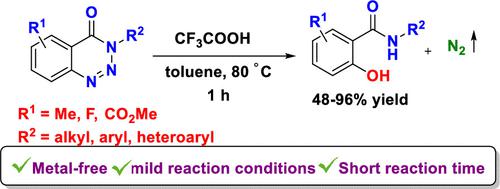
An efficient trifluoroacetic acid-mediated denitrogenative hydroxylation of 1,2,3-benzotriazin-4(3H)-ones is described. This metal-free approach is compatible with a wide range of 1,2,3-benzotriazin-4(3H)-ones, affording ortho-hydroxylated benzamides in good to high yields with a short reaction time. The reaction is believed to proceed via a benzene diazonium intermediate. The synthetic utility of the reaction was successfully demonstrated by the preparation of an antimicrobial drug, Riparin C, and benzoxazine-2,4(3H)-diones in good yields.
中文翻译:

三氟乙酸介导的 1,2,3-Benzotriazin-4(3H)-ones 的脱氮邻羟基化:一种无金属方法
描述了一种有效的三氟乙酸介导的 1,2,3-benzotriazin-4(3 H )-ones 的脱氮羟基化。这种不含金属的方法与多种 1,2,3-benzotriazin-4(3 H )-one相容,可在较短的反应时间内以良好至高产率提供邻羟基化苯甲酰胺。据信该反应通过苯重氮中间体进行。该反应的合成效用通过以良好收率制备抗微生物药物利肝素 C 和苯并恶嗪-2,4(3 H )-二酮类化合物得到成功证明。
更新日期:2022-06-14
中文翻译:

三氟乙酸介导的 1,2,3-Benzotriazin-4(3H)-ones 的脱氮邻羟基化:一种无金属方法
描述了一种有效的三氟乙酸介导的 1,2,3-benzotriazin-4(3 H )-ones 的脱氮羟基化。这种不含金属的方法与多种 1,2,3-benzotriazin-4(3 H )-one相容,可在较短的反应时间内以良好至高产率提供邻羟基化苯甲酰胺。据信该反应通过苯重氮中间体进行。该反应的合成效用通过以良好收率制备抗微生物药物利肝素 C 和苯并恶嗪-2,4(3 H )-二酮类化合物得到成功证明。






























 京公网安备 11010802027423号
京公网安备 11010802027423号