当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Surface Structure Engineering of PtPd Nanoparticles for Boosting Ammonia Oxidation Electrocatalysis
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2022-06-14 , DOI: 10.1021/acsami.2c04711
Zhenzhong Liu 1 , Yi Li 1 , Xiangsong Zhang 1 , Shaosheng Rao 1 , Jinghan Li 1 , Wenlong Wang 1 , Zhongti Sun 1 , Juan Yang 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2022-06-14 , DOI: 10.1021/acsami.2c04711
Zhenzhong Liu 1 , Yi Li 1 , Xiangsong Zhang 1 , Shaosheng Rao 1 , Jinghan Li 1 , Wenlong Wang 1 , Zhongti Sun 1 , Juan Yang 1
Affiliation
|
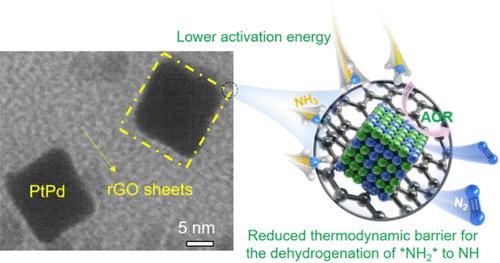
Achieving high catalytic ammonia oxidation reaction (AOR) performance of Pt-based catalysts is of paramount significance for the development of direct ammonia fuel cells (DAFCs). However, the high energy barrier of dehydrogenation of *NH2 to *NH and easy deactivation by *N on the Pt surface make the AOR show sluggish kinetics. Here, we have put forward an alloying and surface modulation tactic to optimize Pt catalysts. Several spherical PtM (M = Co, Ni, Cu, and Pd) binary nanoparticles were controllably loaded on reduced graphene oxide (rGO). Among others, spherical PtPd nanoparticles displayed the most efficient catalytic activity. Further surface engineering of PtPd nanoparticles with a cubic-dominant structure has resulted in dramatic AOR activity improvements. The optimized (100)Pt85Pd15/rGO exhibited a low onset potential (0.467 V vs reversible hydrogen electrode (RHE)) and high peak mass activity (164.9 A g–1), much better than commercial Pt/C. Nevertheless, a short-term stability test along with morphology, structure, and composition characterizations indicate that the leaching of Pd atoms from PtPd alloy nanoparticles, their structure transformations, and the possible poisoning effects by the N-containing intermediates could result in the catalyst’s activity loss during the AOR electrocatalysis. A temperature-dependent electrochemical test confirmed a reduced activation energy (∼12 kJ mol–1 decrease) of cubic-dominant PtPd compared to Pt/C. Density functional theory calculations further demonstrated that Pd atoms in Pt decrease the reaction energy barrier of electrochemical dehydrogenation of *NH2 to *NH, resulting in an excellent catalytic activity for the AOR.
中文翻译:

用于促进氨氧化电催化的 PtPd 纳米颗粒的表面结构工程
实现 Pt 基催化剂的高催化氨氧化反应 (AOR) 性能对于直接氨燃料电池 (DAFC) 的发展至关重要。然而,*NH 2脱氢生成*NH的高能垒和*N在Pt表面容易失活使得AOR表现出缓慢的动力学。在这里,我们提出了一种合金化和表面调节策略来优化 Pt 催化剂。几个球形 PtM(M = Co、Ni、Cu 和 Pd)二元纳米颗粒可控地负载在还原氧化石墨烯 (rGO) 上。其中,球形 PtPd 纳米颗粒表现出最有效的催化活性。具有立方主导结构的 PtPd 纳米粒子的进一步表面工程导致 AOR 活性显着提高。优化(100)Pt 85 Pd 15 /rGO 表现出低起始电位(0.467 V vs 可逆氢电极 (RHE))和高峰值质量活度(164.9 A g –1),远优于商业 Pt/C。然而,短期稳定性测试以及形态、结构和成分表征表明,Pd 原子从 PtPd 合金纳米颗粒中的浸出、它们的结构转变以及含氮中间体可能产生的中毒效应可能会导致催化剂的活性AOR 电催化过程中的损失。与温度相关的电化学测试证实活化能降低(~12 kJ mol –1与 Pt/C 相比,立方主 PtPd 减少)。密度泛函理论计算进一步表明,Pt中的Pd原子降低了*NH 2电化学脱氢为*NH的反应能垒,从而对AOR具有优异的催化活性。
更新日期:2022-06-14
中文翻译:

用于促进氨氧化电催化的 PtPd 纳米颗粒的表面结构工程
实现 Pt 基催化剂的高催化氨氧化反应 (AOR) 性能对于直接氨燃料电池 (DAFC) 的发展至关重要。然而,*NH 2脱氢生成*NH的高能垒和*N在Pt表面容易失活使得AOR表现出缓慢的动力学。在这里,我们提出了一种合金化和表面调节策略来优化 Pt 催化剂。几个球形 PtM(M = Co、Ni、Cu 和 Pd)二元纳米颗粒可控地负载在还原氧化石墨烯 (rGO) 上。其中,球形 PtPd 纳米颗粒表现出最有效的催化活性。具有立方主导结构的 PtPd 纳米粒子的进一步表面工程导致 AOR 活性显着提高。优化(100)Pt 85 Pd 15 /rGO 表现出低起始电位(0.467 V vs 可逆氢电极 (RHE))和高峰值质量活度(164.9 A g –1),远优于商业 Pt/C。然而,短期稳定性测试以及形态、结构和成分表征表明,Pd 原子从 PtPd 合金纳米颗粒中的浸出、它们的结构转变以及含氮中间体可能产生的中毒效应可能会导致催化剂的活性AOR 电催化过程中的损失。与温度相关的电化学测试证实活化能降低(~12 kJ mol –1与 Pt/C 相比,立方主 PtPd 减少)。密度泛函理论计算进一步表明,Pt中的Pd原子降低了*NH 2电化学脱氢为*NH的反应能垒,从而对AOR具有优异的催化活性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号