当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of Host Flexibility on Selectivity in a Supramolecular Host-Catalyzed Enantioselective aza-Darzens Reaction
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-06-14 , DOI: 10.1021/jacs.2c04182 Stephen M Bierschenk 1, 2 , Judy Y Pan 1, 2 , Nicholas S Settineri 2 , Ulrike Warzok 2 , Robert G Bergman 1, 2 , Kenneth N Raymond 1, 2 , F Dean Toste 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-06-14 , DOI: 10.1021/jacs.2c04182 Stephen M Bierschenk 1, 2 , Judy Y Pan 1, 2 , Nicholas S Settineri 2 , Ulrike Warzok 2 , Robert G Bergman 1, 2 , Kenneth N Raymond 1, 2 , F Dean Toste 1, 2
Affiliation
|
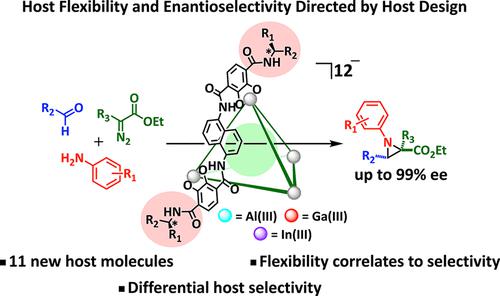
A highly enantioselective aza-Darzens reaction (up to 99% ee) catalyzed by an enantiopure supramolecular host has been discovered. To understand the role of host structure on reaction outcome, nine new gallium(III)-based enantiopure supramolecular assemblies were prepared via substitution of the external chiral amide. Despite the distal nature of the substitution in these catalysts, changes in enantioselectivity (61 to 90% ee) in the aziridine product were observed. The enantioselectivities were correlated to the flexibility of the supramolecular host scaffold as measured by the kinetics of exchange of a model cationic guest. This correlation led to the development of a best-in-class catalyst by substituting the gallium(III)-based host with one based on indium(III), which generated the most flexible and selective catalyst.
中文翻译:

主体灵活性对超分子主体催化的对映选择性氮杂-Darzens 反应选择性的影响
已发现由对映纯超分子主体催化的高度对映选择性 aza-Darzens 反应(高达 99% ee)。为了了解主体结构对反应结果的作用,通过取代外部手性酰胺制备了九种新的基于镓 (III) 的对映体纯超分子组装体。尽管这些催化剂中的取代具有远端性质,但观察到氮丙啶产物的对映选择性(61% 至 90% ee)发生了变化。如通过模型阳离子客体的交换动力学所测量的,对映选择性与超分子宿主支架的灵活性相关。这种相关性导致通过用基于铟(III)的主体替代基于镓(III)的主体来开发同类最佳的催化剂,从而产生最灵活和选择性的催化剂。
更新日期:2022-06-14
中文翻译:

主体灵活性对超分子主体催化的对映选择性氮杂-Darzens 反应选择性的影响
已发现由对映纯超分子主体催化的高度对映选择性 aza-Darzens 反应(高达 99% ee)。为了了解主体结构对反应结果的作用,通过取代外部手性酰胺制备了九种新的基于镓 (III) 的对映体纯超分子组装体。尽管这些催化剂中的取代具有远端性质,但观察到氮丙啶产物的对映选择性(61% 至 90% ee)发生了变化。如通过模型阳离子客体的交换动力学所测量的,对映选择性与超分子宿主支架的灵活性相关。这种相关性导致通过用基于铟(III)的主体替代基于镓(III)的主体来开发同类最佳的催化剂,从而产生最灵活和选择性的催化剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号