当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alkylidene Carbene from Silyl Vinyl Iodide Provides Mechanistic Insights on Trimethylenemethane Diyl-Mediated Tandem Cyclizations
Organic Letters ( IF 4.9 ) Pub Date : 2022-06-14 , DOI: 10.1021/acs.orglett.2c01622 Dong Whan Jee 1 , Jinnie Myung 2 , R Daniel Little 2 , Sunkyu Han 1 , Hee-Yoon Lee 1
Organic Letters ( IF 4.9 ) Pub Date : 2022-06-14 , DOI: 10.1021/acs.orglett.2c01622 Dong Whan Jee 1 , Jinnie Myung 2 , R Daniel Little 2 , Sunkyu Han 1 , Hee-Yoon Lee 1
Affiliation
|
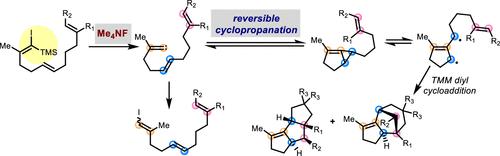
We describe a method to generate alkylidene carbenes via tetramethylammonium-fluoride-induced desilylation of silyl vinyl iodides. The reversible carbene generation from an iodovinyl anion enabled us to unearth mechanistic aspects of the trimethylenemethane (TMM) diyl cyclization reaction that could not be explored via previous methods. We observed that a slow diyl–diylophile cycloaddition can induce the reversible formation of an alkylidene carbene from the TMM diyl intermediate via a retro-cyclopropanation at ambient temperature.
中文翻译:

来自甲硅烷基碘化乙烯的亚烷基卡宾为三亚甲基甲烷二基介导的串联环化提供了机理见解
我们描述了一种通过四甲基氟化铵诱导的甲硅烷基乙烯基碘化物的脱硅烷生成亚烷基卡宾的方法。碘乙烯基阴离子的可逆卡宾生成使我们能够挖掘出三亚甲基甲烷 (TMM) 二基环化反应的机理方面,而这是以前的方法无法探索的。我们观察到,缓慢的二基-亲二基环加成反应可以诱导 TMM 二基中间体在环境温度下通过逆环丙烷化可逆地形成亚烷基卡宾。
更新日期:2022-06-14
中文翻译:

来自甲硅烷基碘化乙烯的亚烷基卡宾为三亚甲基甲烷二基介导的串联环化提供了机理见解
我们描述了一种通过四甲基氟化铵诱导的甲硅烷基乙烯基碘化物的脱硅烷生成亚烷基卡宾的方法。碘乙烯基阴离子的可逆卡宾生成使我们能够挖掘出三亚甲基甲烷 (TMM) 二基环化反应的机理方面,而这是以前的方法无法探索的。我们观察到,缓慢的二基-亲二基环加成反应可以诱导 TMM 二基中间体在环境温度下通过逆环丙烷化可逆地形成亚烷基卡宾。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号