Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2022-06-13 , DOI: 10.1016/j.molliq.2022.119603 Hassan M.A. Hassan , M.R. El-Aassar , Mohammed A. El-Hashemy , Mohamed A. Betiha , Meshal Alzaid , Almaha N. Alqhobisi , Linah A. Alzarea , Ibrahim Hotan Alsohaimi
|
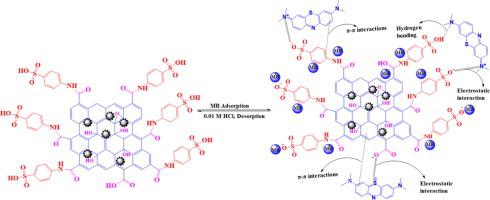
In this work, a superior magnetic graphene oxide (GO) functionalized with sulfanilic acid (Fe3O4@GO@SA) was successfully fabricated by an in-situ precipitation approach and amidation reaction as a robust material for methylene blue dye (MB) removal. The obtained material was investigated by different techniques, such as XRD, FTIR, TGA, SEM, zeta potential analysis, and BET analysis. The surface areas of GO, MGO, and the Fe3O4@GO@SA nanocomposite were measured to be 3.30, 95.2, and 112 m2/g, respectively. The particle size of Fe3O4@GO@SA was found to be 12.8 nm. The adsorption of MB dye under various adsorption parameters, namely, contact time, temperature, dose, pH, and initial concentration of MB was investigated. The findings revealed that Fe3O4@GO@SA possessed good adsorption performance for MB dye under optimal conditions (30.0 mg/dose, 240 min/contact time, pH 8, 298 K/T, and 100 rpm/shaking speed). The adsorption kinetics of Fe3O4@GO@SA obeyed pseudo first-order kinetics, and the Langmuir model was the best matched model for MB removal. The highest uptake capacity (qm) at 298 K was reported to be 317 mg/g. The thermodynamic variables (ΔH°, ΔS°, and ΔG°) suggest that the removal of MB dye using the Fe3O4@GO@SA adsorbent is a feasible, exothermic and physical process. Fe3O4@GO@SA adsorbs MB molecules via three mechanisms including π-π stacking, columbic attraction, and hydrogen bonding. The Fe3O4@GO@SA nanocomposite exhibited good reusability. All the experimental results show that the Fe3O4@GO@SA nanocomposite has potential applications in future environmental management.
中文翻译:

磺胺酸功能化磁性 GO 作为一种强大的吸附剂,可有效吸附水溶液中的亚甲基蓝
在这项工作中,通过原位沉淀法和酰胺化反应成功地制备了一种用磺胺酸(Fe 3 O 4 @GO@SA)功能化的优质磁性氧化石墨烯(GO)作为亚甲基蓝染料(MB)的稳健材料。移动。所获得的材料通过不同的技术进行了研究,如 XRD、FTIR、TGA、SEM、zeta 电位分析和 BET 分析。GO、MGO 和 Fe 3 O的表面积4@GO@SA 纳米复合材料的测量值分别为 3.30、95.2 和 112 m 2 /g。Fe 3 O 4 @GO@SA的粒径为 12.8 nm。研究了MB染料在各种吸附参数下的吸附,即接触时间、温度、剂量、pH和MB初始浓度。结果表明,Fe 3 O 4 @GO@SA 在最佳条件下(30.0 mg/dose、240 min/接触时间、pH 8、298 K/T 和 100 rpm/振摇速度)对 MB 染料具有良好的吸附性能。Fe 3 O 4 @GO@SA的吸附动力学服从准一级动力学,Langmuir模型是MB去除的最佳匹配模型。最高吸收能力(qm ) 在 298 K 时被报道为 317 mg/g。热力学变量(ΔH°、ΔS° 和 ΔG°)表明,使用 Fe 3 O 4 @GO@SA 吸附剂去除 MB 染料是一种可行的放热物理过程。Fe 3 O 4 @GO@SA 通过三种机制吸附 MB 分子,包括 π-π 堆积、钴吸引和氢键。Fe 3 O 4 @GO@SA 纳米复合材料表现出良好的可重复使用性。所有实验结果表明,Fe 3 O 4 @GO@SA 纳米复合材料在未来的环境管理中具有潜在的应用价值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号