当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isolation, Biosynthetic Investigation, and Biological Evaluation of Maniwamycin G, an Azoxyalkene Compound from Streptomyces sp. TOHO-M025
Journal of Natural Products ( IF 3.3 ) Pub Date : 2022-06-11 , DOI: 10.1021/acs.jnatprod.2c00131 Ayaka Tatsukawa 1 , Yu Tanaka 2, 3 , Haruka Nagano 2, 3 , Atsushi Fukumoto 4 , Yojiro Anzai 4 , Kenji Arakawa 1, 2, 3
Journal of Natural Products ( IF 3.3 ) Pub Date : 2022-06-11 , DOI: 10.1021/acs.jnatprod.2c00131 Ayaka Tatsukawa 1 , Yu Tanaka 2, 3 , Haruka Nagano 2, 3 , Atsushi Fukumoto 4 , Yojiro Anzai 4 , Kenji Arakawa 1, 2, 3
Affiliation
|
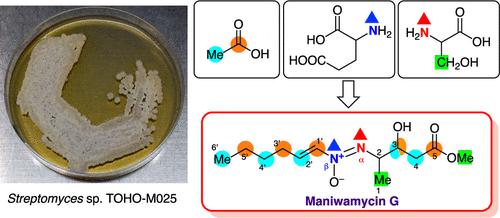
A new maniwamycin analogue, maniwamycin G, was isolated from Streptomyces sp. TOHO-M025 as a major product. Maniwamycin G has a molecular formula of C12H22N2O4, and its extensive NMR analysis revealed that maniwamycin G contains a methoxycarbonyl group instead of an amide as found in maniwamycin F. Its C-2 and C-3 configurations were determined to be (2R, 3R) by circular dichroism spectrum and a modified Mosher method, respectively. The biosynthetic origin of maniwamycin G was investigated using isotope-labeled compounds. The carbon source of maniwamycin G is four acetate units (C-1′, C-2′; C-3′, C-4′; C-5′, C-6′; and C-4, C-5) and l-serine (C-1 to C-3). The nitrogen atom attached at C-2 (Nα) originates from serine, whereas the nitrogen atom of a hexen-1-yl amine unit (Nβ) is derived from glutamic acid. The quorum-sensing inhibitory activity of maniwamycin G was 2-fold lower than that of maniwamycin F.
中文翻译:

Maniwamycin G 的分离、生物合成研究和生物学评价,一种来自 Streptomyces sp. 的 Azoxyalkene 化合物。东宝-M025
从链霉菌中分离出一种新的马尼瓦霉素类似物马尼瓦霉素G。TOHO-M025作为主打产品。Maniwamycin G 的分子式为 C 12 H 22 N 2 O 4,其广泛的 NMR 分析表明,maniwamycin G 含有甲氧羰基而不是 maniwamycin F 中的酰胺。测定了其 C-2 和 C-3 构型通过圆二色光谱和改进的 Mosher 方法分别为 (2 R , 3 R )。使用同位素标记的化合物研究了马尼瓦霉素 G 的生物合成来源。马尼瓦霉素 G 的碳源是四个乙酸酯单元(C-1',C-2';C-3',C-4';C-5',C-6';和 C-4,C-5)和l-丝氨酸(C-1 至 C-3)。连接在 C-2 (Nα) 上的氮原子源自丝氨酸,而 hexen-1-yl amine unit (Nβ) 的氮原子源自谷氨酸。马尼瓦霉素 G 的群体感应抑制活性比马尼瓦霉素 F 低 2 倍。
更新日期:2022-06-11
中文翻译:

Maniwamycin G 的分离、生物合成研究和生物学评价,一种来自 Streptomyces sp. 的 Azoxyalkene 化合物。东宝-M025
从链霉菌中分离出一种新的马尼瓦霉素类似物马尼瓦霉素G。TOHO-M025作为主打产品。Maniwamycin G 的分子式为 C 12 H 22 N 2 O 4,其广泛的 NMR 分析表明,maniwamycin G 含有甲氧羰基而不是 maniwamycin F 中的酰胺。测定了其 C-2 和 C-3 构型通过圆二色光谱和改进的 Mosher 方法分别为 (2 R , 3 R )。使用同位素标记的化合物研究了马尼瓦霉素 G 的生物合成来源。马尼瓦霉素 G 的碳源是四个乙酸酯单元(C-1',C-2';C-3',C-4';C-5',C-6';和 C-4,C-5)和l-丝氨酸(C-1 至 C-3)。连接在 C-2 (Nα) 上的氮原子源自丝氨酸,而 hexen-1-yl amine unit (Nβ) 的氮原子源自谷氨酸。马尼瓦霉素 G 的群体感应抑制活性比马尼瓦霉素 F 低 2 倍。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号