当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tunable Emission Phosphor Ca0.75Sr0.2Mg1.05(Si2O6):Eu2+, Mn2+: Luminescence and Mechanism of Host, Energy Transfer of Eu2+ → Mn2+, Eu2+ → Host, and Host → Mn2+
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-09-02 00:00:00 , DOI: 10.1021/acs.jpcc.6b05993
Yuansheng Sun 1 , Panlai Li 1 , Zhijun Wang 1 , Jinge Cheng 1 , Zhenling Li 1 , Chao Wang 1 , Miaomiao Tian 1 , Zhiping Yang 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-09-02 00:00:00 , DOI: 10.1021/acs.jpcc.6b05993
Yuansheng Sun 1 , Panlai Li 1 , Zhijun Wang 1 , Jinge Cheng 1 , Zhenling Li 1 , Chao Wang 1 , Miaomiao Tian 1 , Zhiping Yang 1
Affiliation
|
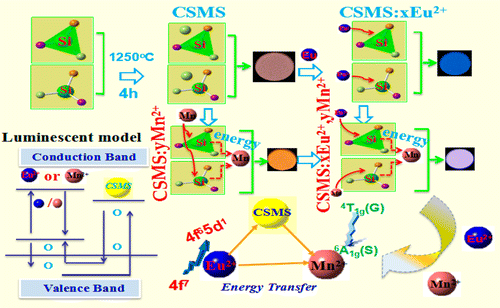
A series of Ca0.75Sr0.2Mg1.05(Si2O6) (CSMS):xEu2+, yMn2+ phosphors were synthesized by a high temperature solid state reaction technique in a reducing atmosphere (1250 °C, 4 h). Under excitation of 270 nm, the emission spectrum of CSMS host is characterized by a broad band with two distinct emission peaks (453 and 580 nm), tailing on the energy side from approximately 350 to 800 nm. Thus, there is an orange-yellow emission in CSMS host. The different trap depths of thermoluminescence spectra (E1 = 0.6453 eV, E2 = 0.8271 eV) are measured and these traps will fade away when Eu2+ or Mn2+ ions are doped. In addition, the blue-shift, red-shift, and intensity changes of temperature dependence emission spectra in Ca0.75Sr0.2Mg1.05(Si2O6) host are observed with higher temperature. For Eu2+ single doped CSMS, which has an obvious absorption in the near-ultraviolet and blue region with a wide band of excitation spectrum ranging from 200 to 450 nm monitored at 453 nm. And the emission spectrum presents an obvious red-shift phenomenon. Mn2+ single-doped CSMS presents the weak emission intensity at 680 nm with the excitation of 415 nm. For Eu2+ and Mn2+ codoped, the energy transfer of Eu2+ → Mn2+, Eu2+ → host, and host → Mn2+ can be demonstrated, and there should be a blue-white emission in CSMS:0.03Eu2+, 0.02Mn2+ with CIE (0.2682, 0.2056). In short, the CSMS host presents a good application prospect in near-ultraviolet trichromatic field of white LEDs, and the luminescence properties of CSMS:xEu2+, yMn2+ phosphors are particularly worthy of academic investigation.
中文翻译:

可调谐发射磷Ca 0.75 Sr 0.2 Mg 1.05(Si 2 O 6):Eu 2 +,Mn 2+:主体的发光和机理,Eu 2 + →Mn 2 +,Eu 2 + →主体和主体→的能量转移锰2+
通过高温固态反应技术在还原气氛(1250°C,4 h)下合成了一系列Ca 0.75 Sr 0.2 Mg 1.05(Si 2 O 6)(CSMS):x Eu 2 +,y Mn 2+荧光粉)。在270 nm的激发下,CSMS主机的发射光谱的特征是具有两个不同发射峰(453和580 nm)的宽带,在能量侧拖尾大约为350至800 nm。因此,CSMS主机中会发出橙黄色的光。热发光光谱的不同陷阱深度(E 1 = 0.6453 eV,E 2= 0.8271 eV),并且当掺杂Eu 2+或Mn 2+离子时,这些陷阱会消失。另外,在较高温度下观察到Ca 0.75 Sr 0.2 Mg 1.05(Si 2 O 6)主体中的温度依赖性发射光谱的蓝移,红移和强度变化。对于Eu 2+单掺杂CSMS,它在近紫外和蓝色区域具有明显的吸收,并在453 nm处监测到200至450 nm的宽激发光谱。发射光谱呈现明显的红移现象。锰2+单掺杂CSMS在680 nm处激发强度为415 nm时呈现出较弱的发射强度。对于共掺杂的Eu 2+和Mn 2+,可以证明Eu 2 + →Mn 2 +,Eu 2 + →主体和主体→Mn 2+的能量转移,并且在CSMS中应该有蓝白色发射: 0.03Eu 2 +,0.02Mn 2+和CIE(0.2682,0.2056 )。简而言之,CSMS主机在白色LED的近紫外三基色领域具有良好的应用前景,CSMS的发光特性:x Eu 2 +,y Mn 2+荧光粉特别值得学术研究。
更新日期:2016-09-02
中文翻译:

可调谐发射磷Ca 0.75 Sr 0.2 Mg 1.05(Si 2 O 6):Eu 2 +,Mn 2+:主体的发光和机理,Eu 2 + →Mn 2 +,Eu 2 + →主体和主体→的能量转移锰2+
通过高温固态反应技术在还原气氛(1250°C,4 h)下合成了一系列Ca 0.75 Sr 0.2 Mg 1.05(Si 2 O 6)(CSMS):x Eu 2 +,y Mn 2+荧光粉)。在270 nm的激发下,CSMS主机的发射光谱的特征是具有两个不同发射峰(453和580 nm)的宽带,在能量侧拖尾大约为350至800 nm。因此,CSMS主机中会发出橙黄色的光。热发光光谱的不同陷阱深度(E 1 = 0.6453 eV,E 2= 0.8271 eV),并且当掺杂Eu 2+或Mn 2+离子时,这些陷阱会消失。另外,在较高温度下观察到Ca 0.75 Sr 0.2 Mg 1.05(Si 2 O 6)主体中的温度依赖性发射光谱的蓝移,红移和强度变化。对于Eu 2+单掺杂CSMS,它在近紫外和蓝色区域具有明显的吸收,并在453 nm处监测到200至450 nm的宽激发光谱。发射光谱呈现明显的红移现象。锰2+单掺杂CSMS在680 nm处激发强度为415 nm时呈现出较弱的发射强度。对于共掺杂的Eu 2+和Mn 2+,可以证明Eu 2 + →Mn 2 +,Eu 2 + →主体和主体→Mn 2+的能量转移,并且在CSMS中应该有蓝白色发射: 0.03Eu 2 +,0.02Mn 2+和CIE(0.2682,0.2056 )。简而言之,CSMS主机在白色LED的近紫外三基色领域具有良好的应用前景,CSMS的发光特性:x Eu 2 +,y Mn 2+荧光粉特别值得学术研究。








































 京公网安备 11010802027423号
京公网安备 11010802027423号