Cellular Signalling ( IF 4.4 ) Pub Date : 2022-06-09 , DOI: 10.1016/j.cellsig.2022.110378
Chan Wen 1 , Chen Wang 2 , Conghui Hu 1 , Tiantian Qi 1 , Ruihua Jing 3 , Yunqing Wang 1 , Ming Zhang 1 , Yongping Shao 4 , Cheng Pei 1
|
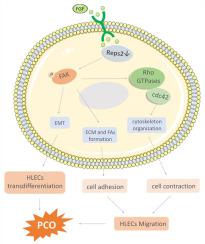
Posterior capsular opacification (PCO) can cause postoperative visual loss after cataract surgery. Residual human lens epithelial cell (HLEC) proliferation, migration, epithelial-mesenchymal transition (EMT) and synthesis of extracellular matrix (ECM) are the entitative reasons for PCO. Low expression of Ral-binding protein 1-associated Eps domain-containing 2 (REPS2) and high levels of basic fibroblast growth factor (b-FGF) were observed in the lens and postoperative aqueous humor of cataract patients. REPS2 was identified as a negative regulator in growth factor signaling; however, its function in HLECs is unknown. This was first investigated in the present study by evaluating REPS2 expression in anterior lens capsules from cataract patients, a mouse cataract model, and HLE-b3 cells. The biological function of REPS2 in HLE-B3 cells was assessed by REPS2 silencing and Cell Counting Kit 8, wound healing, Transwell migration, F-actin staining, G-protein pulldown and western blot assays. In the present study, REPS2 was significantly downregulated in human and mouse cataract capsules and H2O2-treated HLE-B3 cells. REPS2 knockdown increased fibronectin, type I collagen, and α-smooth muscle actin expression levels and stimulated HLECs proliferation and migration; these effects were enhanced by FGF treatment and accompanied with focal adhesion kinase (FAK) phosphorylation, cell division cycle 42 (Cdc42) activation, focal adhesion protein upregulation, and F-actin cytoskeleton reorganization. However, treatment with the FAK inhibitor PF573228 abolished these effects. Thus, REPS2 downregulation in cataract HLECs induces their proliferation and facilitates FGF-induced ECM synthesis, EMT, cell adhesion and migration by activating FAK/Cdc42 signaling, which may underlie PCO pathogenesis.
中文翻译:

REPS2 下调通过 FAK/Cdc42 信号传导促进 FGF 诱导的人晶状体上皮细胞粘附和迁移,并有助于后囊混浊
后囊膜混浊(PCO)可导致白内障术后视力丧失。残留的人晶状体上皮细胞 (HLEC) 增殖、迁移、上皮间质转化 (EMT) 和细胞外基质 (ECM) 的合成是 PCO 的实体原因。在白内障患者的晶状体和术后房水中观察到 Ral 结合蛋白 1 相关 Eps 结构域 2 (REPS2) 的低表达和高水平的碱性成纤维细胞生长因子 (b-FGF)。REPS2 被确定为生长因子信号传导的负调节剂;然而,它在 HLEC 中的功能尚不清楚。本研究首先通过评估白内障患者、小鼠白内障模型和 HLE-b3 细胞的晶状体前囊中的 REPS2 表达来研究这一点。REPS2沉默和细胞计数试剂盒 8、伤口愈合、Transwell 迁移、F-肌动蛋白染色、G 蛋白 pulldown 和蛋白质印迹分析。在本研究中,REPS2 在人和小鼠白内障胶囊和 H 2 O 2处理的 HLE-B3 细胞中显着下调。REPS2敲低增加了纤连蛋白、I 型胶原蛋白和 α-平滑肌肌动蛋白的表达水平,并刺激了 HLECs 的增殖和迁移;FGF 处理增强了这些作用,并伴随着粘着斑激酶 (FAK) 磷酸化、细胞分裂周期 42 (Cdc42) 激活、粘着斑蛋白上调和 F-肌动蛋白细胞骨架重组。然而,用 FAK 抑制剂 PF573228 治疗消除了这些影响。因此,白内障 HLEC 中的 REPS2 下调诱导其增殖并通过激活 FAK/Cdc42 信号传导促进 FGF 诱导的 ECM 合成、EMT、细胞粘附和迁移,这可能是 PCO 发病机制的基础。

































 京公网安备 11010802027423号
京公网安备 11010802027423号