当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Substituted Pyridines via Formal (3+3) Cycloaddition of Enamines with Unsaturated Aldehydes and Ketones
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-06-09 , DOI: 10.1021/acs.joc.2c00576 Xi-Jie Dai 1 , Paul Krolikowski 1 , James I Murray 2 , Carolyn S Wei 2 , Peter K Dornan 1 , Andreas R Rötheli 1 , Seb Caille 2 , Oliver R Thiel 1 , Austin G Smith 2 , Andrew T Parsons 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-06-09 , DOI: 10.1021/acs.joc.2c00576 Xi-Jie Dai 1 , Paul Krolikowski 1 , James I Murray 2 , Carolyn S Wei 2 , Peter K Dornan 1 , Andreas R Rötheli 1 , Seb Caille 2 , Oliver R Thiel 1 , Austin G Smith 2 , Andrew T Parsons 1
Affiliation
|
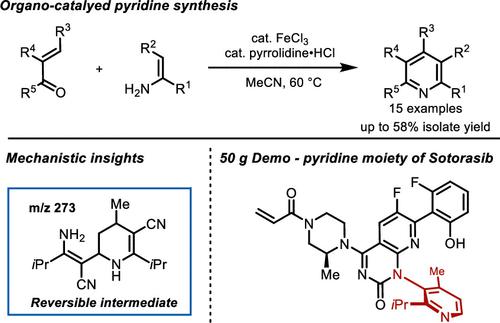
An organocatalyzed, formal (3+3) cycloaddition reaction is described for the practical synthesis of substituted pyridines. Starting from readily available enamines and enal/ynal/enone substrates, the protocol affords tri- or tetrasubstituted pyridine scaffolds bearing various functional groups. This method was demonstrated on a 50 g scale, enabling the synthesis of 2-isopropyl-4-methylpyridin-3-amine, a raw material used for the manufacture of sotorasib. Mechanistic analysis using two-dimensional nuclear magnetic resonance (NMR) spectrometry revealed the transformation proceeds through the reversible formation of a stable reaction off-cycle species that precedes pyridine formation. In situ reaction progress kinetic analysis and control NMR studies were employed to better understand the role of FeCl3 and pyrrolidine hydrochloride in promoting the reaction.
中文翻译:

烯胺与不饱和醛和酮的形式 (3+3) 环加成合成取代吡啶
描述了一种有机催化的形式 (3+3) 环加成反应,用于实际合成取代的吡啶。从现成的烯胺和烯/烯/烯酮底物开始,该协议提供了带有各种官能团的三或四取代吡啶支架。该方法在 50 g 规模上进行了演示,能够合成 2-isopropyl-4-methylpyridin-3-amine,这是一种用于制造 sotorasib 的原材料。使用二维核磁共振 (NMR) 光谱法进行的机理分析表明,转化过程是通过在吡啶形成之前可逆地形成稳定的非循环反应物质来进行的。采用原位反应进展动力学分析和对照 NMR 研究来更好地了解 FeCl 的作用3与盐酸吡咯烷促进反应。
更新日期:2022-06-09
中文翻译:

烯胺与不饱和醛和酮的形式 (3+3) 环加成合成取代吡啶
描述了一种有机催化的形式 (3+3) 环加成反应,用于实际合成取代的吡啶。从现成的烯胺和烯/烯/烯酮底物开始,该协议提供了带有各种官能团的三或四取代吡啶支架。该方法在 50 g 规模上进行了演示,能够合成 2-isopropyl-4-methylpyridin-3-amine,这是一种用于制造 sotorasib 的原材料。使用二维核磁共振 (NMR) 光谱法进行的机理分析表明,转化过程是通过在吡啶形成之前可逆地形成稳定的非循环反应物质来进行的。采用原位反应进展动力学分析和对照 NMR 研究来更好地了解 FeCl 的作用3与盐酸吡咯烷促进反应。






























 京公网安备 11010802027423号
京公网安备 11010802027423号