当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Novel and Practical Synthesis of Mavorixafor
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-06-09 , DOI: 10.1021/acs.oprd.2c00076 Weiyuan Liu 1 , Siju Bi 1 , Ting Tian 1 , Ting Zhou 1 , Kuaile Lin 1 , Weicheng Zhou 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-06-09 , DOI: 10.1021/acs.oprd.2c00076 Weiyuan Liu 1 , Siju Bi 1 , Ting Tian 1 , Ting Zhou 1 , Kuaile Lin 1 , Weicheng Zhou 1
Affiliation
|
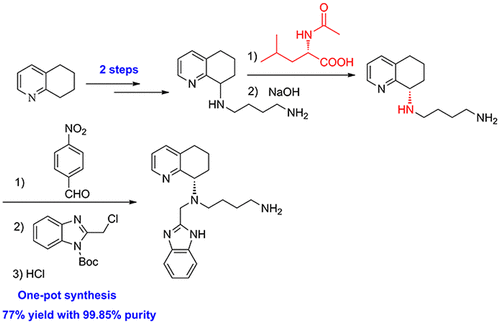
A novel and practical synthesis of mavorixafor (1) is reported. The novelty of this synthetic route is the use of 8-chloro-5,6,7,8-tetrahydroquinoline (9) and 1,4-diaminobutane as the materials, instead of 8-amino-5,6,7,8-tetrahydroquinoline (4) and N,N-diprotected aminobutyraldehyde (6a or 6b). The preparation of (S)-8-(4-aminobutylamino)-5,6,7,8-tetrahydroquinoline (13) by resolution with N-acetyl-l-leucine was first achieved. Then the one-pot synthesis of 1 from 13 involving protection, condensation, and subsequent hydrolysis was successfully developed. In addition, the final product with a satisfactory purity (>99.5%, detected by both achiral and chiral HPLC) was obtained by a simple operation (salification) without column chromatographic purification.
中文翻译:

Mavorixafor 的一种新颖实用的合成方法
报道了一种新颖实用的 mavorixafor ( 1 ) 合成方法。该合成路线的新颖之处在于以8-氯-5,6,7,8-四氢喹啉(9)和1,4-二氨基丁烷为原料,代替了8-氨基-5,6,7,8-四氢喹啉 ( 4 ) 和 N,N-二保护氨基丁醛 ( 6a或6b )。首次实现了N-乙酰-l-亮氨酸拆分制备( S )-8-(4-氨基丁基氨基)-5,6,7,8-四氢喹啉( 13 )。然后从13一锅合成1成功开发了涉及保护、缩合和后续水解的技术。此外,通过简单的操作(成盐),无需柱色谱纯化,即可获得纯度令人满意的最终产品(>99.5%,通过非手性和手性 HPLC 检测)。
更新日期:2022-06-09
中文翻译:

Mavorixafor 的一种新颖实用的合成方法
报道了一种新颖实用的 mavorixafor ( 1 ) 合成方法。该合成路线的新颖之处在于以8-氯-5,6,7,8-四氢喹啉(9)和1,4-二氨基丁烷为原料,代替了8-氨基-5,6,7,8-四氢喹啉 ( 4 ) 和 N,N-二保护氨基丁醛 ( 6a或6b )。首次实现了N-乙酰-l-亮氨酸拆分制备( S )-8-(4-氨基丁基氨基)-5,6,7,8-四氢喹啉( 13 )。然后从13一锅合成1成功开发了涉及保护、缩合和后续水解的技术。此外,通过简单的操作(成盐),无需柱色谱纯化,即可获得纯度令人满意的最终产品(>99.5%,通过非手性和手性 HPLC 检测)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号