当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sc(OTf)3/BF3·OEt2-Catalyzed Annulation of 3-Formylchromones with Functionalized Alkenes: Access to Diverse 2-Hydroxybenzophenones
Organic Letters ( IF 4.9 ) Pub Date : 2022-06-09 , DOI: 10.1021/acs.orglett.2c01538 Sabera Sultana 1 , Peter Yuosef M Rubio 1 , Hari Datta Khanal 1 , Yong Rok Lee 1
Organic Letters ( IF 4.9 ) Pub Date : 2022-06-09 , DOI: 10.1021/acs.orglett.2c01538 Sabera Sultana 1 , Peter Yuosef M Rubio 1 , Hari Datta Khanal 1 , Yong Rok Lee 1
Affiliation
|
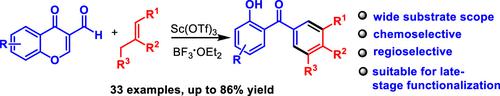
The Sc(OTf)3/BF3·OEt2-catalyzed annulation of 3-formylchromones with functionalized alkenes for the direct construction of 2-hydroxybenzophenones is described. Sc(OTf)3/BF3·OEt2 acts as a synergistic catalyst, providing rapid synthetic access to diversely and highly functionalized 2-hydroxybenzophenones. This reaction has excellent regio- and chemoselectivities and is suitable for late-stage functionalization. The reaction proceeds via [3 + 3] and [4 + 2] cycloaddition processes, through carbonyl-ene, Diels–Alder, or aldol-type reactions. Furthermore, this protocol tolerates the various functional groups present in natural terpenes and steroids.
中文翻译:

Sc(OTf)3/BF3·OEt2 催化 3-甲酰基色酮与官能化烯烃的环化:获得多种 2-羟基二苯甲酮
描述了 Sc(OTf) 3 /BF 3 ·OEt 2催化的 3-甲酰基色酮与官能化烯烃的环化,用于直接构建 2-羟基二苯甲酮。Sc(OTf) 3 /BF 3 ·OEt 2作为一种协同催化剂,可以快速合成多种高度官能化的 2-羟基二苯甲酮。该反应具有优异的区域和化学选择性,适用于后期功能化。该反应通过 [3 + 3] 和 [4 + 2] 环加成过程,通过羰基-烯、Diels-Alder 或醛醇型反应进行。此外,该协议可以容忍天然萜烯和类固醇中存在的各种官能团。
更新日期:2022-06-09
中文翻译:

Sc(OTf)3/BF3·OEt2 催化 3-甲酰基色酮与官能化烯烃的环化:获得多种 2-羟基二苯甲酮
描述了 Sc(OTf) 3 /BF 3 ·OEt 2催化的 3-甲酰基色酮与官能化烯烃的环化,用于直接构建 2-羟基二苯甲酮。Sc(OTf) 3 /BF 3 ·OEt 2作为一种协同催化剂,可以快速合成多种高度官能化的 2-羟基二苯甲酮。该反应具有优异的区域和化学选择性,适用于后期功能化。该反应通过 [3 + 3] 和 [4 + 2] 环加成过程,通过羰基-烯、Diels-Alder 或醛醇型反应进行。此外,该协议可以容忍天然萜烯和类固醇中存在的各种官能团。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号