当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Basis of Huntingtin Fibril Polymorphism Revealed by Cryogenic Electron Microscopy of Exon 1 HTT Fibrils
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-06-09 , DOI: 10.1021/jacs.2c00509 Sergey Nazarov 1, 2 , Anass Chiki 1 , Driss Boudeffa 1 , Hilal A Lashuel 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-06-09 , DOI: 10.1021/jacs.2c00509 Sergey Nazarov 1, 2 , Anass Chiki 1 , Driss Boudeffa 1 , Hilal A Lashuel 1
Affiliation
|
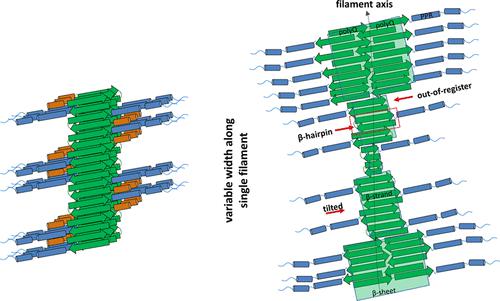
The lack of detailed insight into the structure of aggregates formed by the huntingtin protein (HTT) has hampered the efforts to develop therapeutics and diagnostics targeting pathology formation in the brain of patients with Huntington’s disease. To address this knowledge gap, we investigated the structural properties of in vitro-generated fibrils from exon1 of the huntingtin protein by cryogenic electron microscopy and single-particle analyses. We show that wildtype and mutant exon1 of the huntingtin protein form nonhelical fibrils with a polyglutamine amyloid core composed of β-hairpins with unique characteristics that have not been previously observed with other amyloid filaments. The stacks of β-hairpins form long planar β-sheets (protofilaments) which combine inter- and intra-molecular interactions, with variable stacking angles and occasional out-of-register states of individual β-hairpins. These features and the propensity of protofilaments to undergo lateral association result in a high degree of fibril polymorphisms, including fibrils composed of varying numbers of protofilaments. Our results allow us to speculate on how the flanking domains are organized around the polyglutamine core of the fibril and provide insight into how they might affect the huntingtin fibril structure and polymorphism. The removal of the first 17 amino acids at the N-terminus resulted in surprising intra-fibril structural heterogeneity and reduced fibril’s propensity to lateral associations. Overall, this work provides valuable insights that could help guide future mechanistic studies to elucidate the sequence and structural determinants of huntingtin aggregation, as well as for cryo-EM and structural studies of fibrils derived from huntingtin protein and other disease-associated polyglutamine-containing proteins.
中文翻译:

外显子 1 HTT 原纤维的低温电子显微镜揭示亨廷顿蛋白原纤维多态性的结构基础
缺乏对亨廷顿蛋白 (HTT) 形成的聚集体结构的详细了解阻碍了开发针对亨廷顿病患者大脑中病理形成的治疗和诊断方法的努力。为了解决这一知识空白,我们通过低温电子显微镜和单粒子分析研究了亨廷顿蛋白外显子 1 体外产生的原纤维的结构特性。我们显示亨廷顿蛋白的野生型和突变外显子1 形成非螺旋原纤维,其具有由β-发夹组成的聚谷氨酰胺淀粉样蛋白核心,具有以前在其他淀粉样蛋白丝中未观察到的独特特征。β-发夹的堆叠形成长的平面β-折叠(原丝),结合了分子间和分子内的相互作用,具有可变堆叠角度和个别β-发夹的偶尔失准状态。这些特征和原丝进行横向缔合的倾向导致高度的原纤维多态性,包括由不同数量的原丝组成的原纤维。我们的结果使我们能够推测侧翼结构域是如何围绕原纤维的聚谷氨酰胺核心组织的,并提供了它们如何影响亨廷顿原纤维结构和多态性的见解。N 末端前 17 个氨基酸的去除导致了令人惊讶的原纤维内结构异质性并降低了原纤维横向结合的倾向。总的来说,这项工作提供了有价值的见解,可以帮助指导未来的机制研究,以阐明亨廷顿蛋白聚集的序列和结构决定因素,
更新日期:2022-06-09
中文翻译:

外显子 1 HTT 原纤维的低温电子显微镜揭示亨廷顿蛋白原纤维多态性的结构基础
缺乏对亨廷顿蛋白 (HTT) 形成的聚集体结构的详细了解阻碍了开发针对亨廷顿病患者大脑中病理形成的治疗和诊断方法的努力。为了解决这一知识空白,我们通过低温电子显微镜和单粒子分析研究了亨廷顿蛋白外显子 1 体外产生的原纤维的结构特性。我们显示亨廷顿蛋白的野生型和突变外显子1 形成非螺旋原纤维,其具有由β-发夹组成的聚谷氨酰胺淀粉样蛋白核心,具有以前在其他淀粉样蛋白丝中未观察到的独特特征。β-发夹的堆叠形成长的平面β-折叠(原丝),结合了分子间和分子内的相互作用,具有可变堆叠角度和个别β-发夹的偶尔失准状态。这些特征和原丝进行横向缔合的倾向导致高度的原纤维多态性,包括由不同数量的原丝组成的原纤维。我们的结果使我们能够推测侧翼结构域是如何围绕原纤维的聚谷氨酰胺核心组织的,并提供了它们如何影响亨廷顿原纤维结构和多态性的见解。N 末端前 17 个氨基酸的去除导致了令人惊讶的原纤维内结构异质性并降低了原纤维横向结合的倾向。总的来说,这项工作提供了有价值的见解,可以帮助指导未来的机制研究,以阐明亨廷顿蛋白聚集的序列和结构决定因素,































 京公网安备 11010802027423号
京公网安备 11010802027423号