当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rational Design, Synthesis, and Biological Evaluation of Fluorine- and Chlorine-Substituted Pyrazol-5-yl-benzamide Derivatives as Potential Succinate Dehydrogenase Inhibitors
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2022-06-08 , DOI: 10.1021/acs.jafc.2c01901 Wei Wang 1 , Jianhua Wang 1 , Jipeng Wu 1 , Mengyun Jin 1 , Junling Li 1 , Shiyang Jin 1 , Wangxiang Li 1 , Dan Xu 2, 3 , Xili Liu 1, 3 , Gong Xu 1, 3
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2022-06-08 , DOI: 10.1021/acs.jafc.2c01901 Wei Wang 1 , Jianhua Wang 1 , Jipeng Wu 1 , Mengyun Jin 1 , Junling Li 1 , Shiyang Jin 1 , Wangxiang Li 1 , Dan Xu 2, 3 , Xili Liu 1, 3 , Gong Xu 1, 3
Affiliation
|
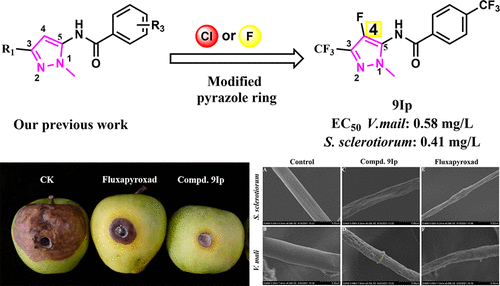
To develop novel succinate dehydrogenase inhibitors (SDHIs), two series of novel N-4-fluoro-pyrazol-5-yl-benzamide and N-4-chloro-pyrazol-5-yl-benzamide derivatives were designed and synthesized, and their antifungal activities were evaluated against Valsa mali, Sclerotinia sclerotiorum, FusaHum graminearum Sehw, Physalospora piricola, and Botrytis cinerea. The bioassay results showed that some of the target compounds exhibited good antifungal activities in vitro against V. mali and S. sclerotiorum. Remarkably, compound 9Ip displayed good in vitro activity against V. mali with an EC50 value of 0.58 mg/L. This outcome was 21-fold greater than that of fluxapyroxad (12.45 mg/L) and close to that of the commercial fungicide tebuconazole (EC50 = 0.36 mg/L). In addition, in vivo experiments proved that compound 9Ip has good protective fungicidal activity with an inhibitory rate of 93.2% against V. mali at 50 mg/L, which was equivalent to that of the positive control tebuconazole (95.5%). The results of molecular docking indicated that there were obvious hydrogen bonds and p−π interactions between compound 9Ip and succinate dehydrogenase (SDH), which could explain the probable action mechanism. In addition, the SDH enzymatic inhibition assay was carried out to further prove its mode of action. Our studies suggest that compound 9Ip could be a fungicidal lead to discover more potent SDHIs for crop protection.
中文翻译:

氟和氯取代的吡唑-5-基苯甲酰胺衍生物作为潜在的琥珀酸脱氢酶抑制剂的合理设计、合成和生物学评价
为开发新型琥珀酸脱氢酶抑制剂(SDHIs),设计合成了两个系列的新型N -4-fluoro-pyrazol-5-yl-benzamide 和N -4-chloro-pyrazol-5-yl-benzamide 衍生物,及其抗真菌剂对Valsa mali、Sclerotinia sclerotiorum、FusaHum graminearum Sehw、 Physalospora piricola和Botrytis cinerea的活性进行了评估。生物测定结果表明,一些目标化合物在体外对马里弧菌和核盘菌具有良好的抗真菌活性。值得注意的是,化合物9Ip表现良好对马里弧菌的体外活性,EC 50值为 0.58 mg/L。这一结果是fluxapyroxad (12.45 mg/L)的21倍,接近商业杀菌剂戊唑醇(EC 50 = 0.36 mg/L)。此外,体内实验证明,化合物9Ip具有良好的保护性杀菌活性,在50 mg/L时对马里疟原虫的抑菌率为93.2% ,与阳性对照戊唑醇(95.5%)相当。分子对接结果表明化合物9Ip之间存在明显的氢键和p-π相互作用和琥珀酸脱氢酶(SDH),这可以解释可能的作用机制。此外,还进行了SDH酶抑制试验以进一步证明其作用方式。我们的研究表明,化合物9Ip可能是一种杀菌剂,可用于发现更有效的作物保护 SDHI。
更新日期:2022-06-08
中文翻译:

氟和氯取代的吡唑-5-基苯甲酰胺衍生物作为潜在的琥珀酸脱氢酶抑制剂的合理设计、合成和生物学评价
为开发新型琥珀酸脱氢酶抑制剂(SDHIs),设计合成了两个系列的新型N -4-fluoro-pyrazol-5-yl-benzamide 和N -4-chloro-pyrazol-5-yl-benzamide 衍生物,及其抗真菌剂对Valsa mali、Sclerotinia sclerotiorum、FusaHum graminearum Sehw、 Physalospora piricola和Botrytis cinerea的活性进行了评估。生物测定结果表明,一些目标化合物在体外对马里弧菌和核盘菌具有良好的抗真菌活性。值得注意的是,化合物9Ip表现良好对马里弧菌的体外活性,EC 50值为 0.58 mg/L。这一结果是fluxapyroxad (12.45 mg/L)的21倍,接近商业杀菌剂戊唑醇(EC 50 = 0.36 mg/L)。此外,体内实验证明,化合物9Ip具有良好的保护性杀菌活性,在50 mg/L时对马里疟原虫的抑菌率为93.2% ,与阳性对照戊唑醇(95.5%)相当。分子对接结果表明化合物9Ip之间存在明显的氢键和p-π相互作用和琥珀酸脱氢酶(SDH),这可以解释可能的作用机制。此外,还进行了SDH酶抑制试验以进一步证明其作用方式。我们的研究表明,化合物9Ip可能是一种杀菌剂,可用于发现更有效的作物保护 SDHI。

































 京公网安备 11010802027423号
京公网安备 11010802027423号