当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tuning the Drug Release from Antibacterial Polycaprolactone/Rifampicin-Based Core–Shell Electrospun Membranes: A Proof of Concept
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2022-06-07 , DOI: 10.1021/acsami.2c04849 Martina Gruppuso 1 , Benedetta Guagnini 1 , Luigi Musciacchio 1 , Francesca Bellemo 2 , Gianluca Turco 1 , Davide Porrelli 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2022-06-07 , DOI: 10.1021/acsami.2c04849 Martina Gruppuso 1 , Benedetta Guagnini 1 , Luigi Musciacchio 1 , Francesca Bellemo 2 , Gianluca Turco 1 , Davide Porrelli 1
Affiliation
|
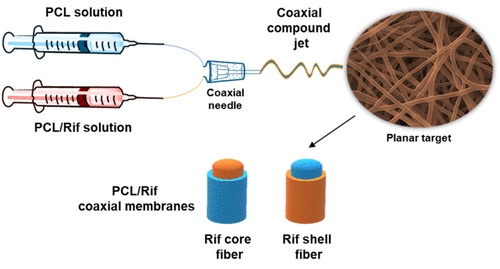
The employment of coaxial fibers for guided tissue regeneration can be extremely advantageous since they allow the functionalization with bioactive compounds to be preserved and released with a long-term efficacy. Antibacterial coaxial membranes based on poly-ε-caprolactone (PCL) and rifampicin (Rif) were synthesized here, by analyzing the effects of loading the drug within the core or on the shell layer with respect to non-coaxial matrices. The membranes were, therefore, characterized for their surface properties in addition to analyzing drug release, antibacterial efficacy, and biocompatibility. The results showed that the lower drug surface density in coaxial fibers hinders the interaction with serum proteins, resulting in a hydrophobic behavior compared to non-coaxial mats. The air-plasma treatment increased their hydrophilicity, although it induced rifampicin degradation. Moreover, the substantially lower release of coaxial fibers influenced the antibacterial efficacy, tested against Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa. Indeed, the coaxial matrices were inhibitory and bactericidal only against S. aureus, while the higher release from non-coaxial mats rendered them active even against E. coli. The biocompatibility of the released rifampicin was assessed too on murine fibroblasts, revealing no cytotoxic effects. Hence, the presented coaxial system should be further optimized to tune the drug release according to the antibacterial effectiveness.
中文翻译:

调节抗菌聚己内酯/利福平核壳电纺膜的药物释放:概念验证
使用同轴纤维引导组织再生可能非常有利,因为它们允许生物活性化合物的功能化得以保存和释放,具有长期功效。通过分析将药物加载到核内或壳层上对非同轴基质的影响,在此合成了基于聚-ε-己内酯 (PCL) 和利福平 (Rif) 的抗菌同轴膜。因此,除了分析药物释放、抗菌功效和生物相容性外,还对膜的表面特性进行了表征。结果表明,同轴纤维中较低的药物表面密度阻碍了与血清蛋白的相互作用,导致与非同轴垫相比的疏水行为。空气等离子处理增加了它们的亲水性,尽管它会诱导利福平降解。此外,显着降低的同轴纤维释放影响了抗菌效果,针对大肠杆菌、金黄色葡萄球菌和铜绿假单胞菌。事实上,同轴基质仅对金黄色葡萄球菌具有抑制和杀菌作用,而非同轴基质的更高释放使它们甚至对大肠杆菌也有活性。释放的利福平的生物相容性也在小鼠成纤维细胞上进行了评估,没有显示出细胞毒性作用。因此,应进一步优化所提出的同轴系统,以根据抗菌效果调整药物释放。
更新日期:2022-06-07
中文翻译:

调节抗菌聚己内酯/利福平核壳电纺膜的药物释放:概念验证
使用同轴纤维引导组织再生可能非常有利,因为它们允许生物活性化合物的功能化得以保存和释放,具有长期功效。通过分析将药物加载到核内或壳层上对非同轴基质的影响,在此合成了基于聚-ε-己内酯 (PCL) 和利福平 (Rif) 的抗菌同轴膜。因此,除了分析药物释放、抗菌功效和生物相容性外,还对膜的表面特性进行了表征。结果表明,同轴纤维中较低的药物表面密度阻碍了与血清蛋白的相互作用,导致与非同轴垫相比的疏水行为。空气等离子处理增加了它们的亲水性,尽管它会诱导利福平降解。此外,显着降低的同轴纤维释放影响了抗菌效果,针对大肠杆菌、金黄色葡萄球菌和铜绿假单胞菌。事实上,同轴基质仅对金黄色葡萄球菌具有抑制和杀菌作用,而非同轴基质的更高释放使它们甚至对大肠杆菌也有活性。释放的利福平的生物相容性也在小鼠成纤维细胞上进行了评估,没有显示出细胞毒性作用。因此,应进一步优化所提出的同轴系统,以根据抗菌效果调整药物释放。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号