当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pd/Et3N·HI-Catalyzed Intramolecular C–H Alkylation to Access [a]-Annulated Indoles via Highly Regioselective Ring-Opening of Epoxides
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-06-07 , DOI: 10.1021/acs.joc.2c00630
Xu Dong 1 , Xi Sun 1 , Yi Yang 1 , Weiwei Liu 1 , Bowen Zheng 1 , Xinjin Li 1 , Daopeng Zhang 1 , Fagang Wang 1 , Hui Liu 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-06-07 , DOI: 10.1021/acs.joc.2c00630
Xu Dong 1 , Xi Sun 1 , Yi Yang 1 , Weiwei Liu 1 , Bowen Zheng 1 , Xinjin Li 1 , Daopeng Zhang 1 , Fagang Wang 1 , Hui Liu 1
Affiliation
|
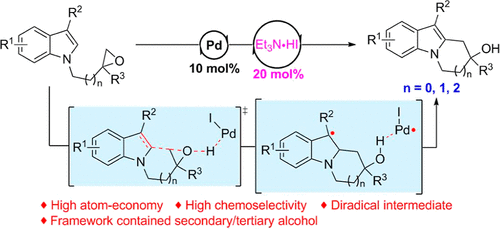
A Pd/Et3N·HI-catalyzed intramolecular C–H alkylation of indoles with epoxides was achieved to furnish N-fused indole frameworks (5-, 6-, and 7-membered rings) bearing an alcohol group. The conversion proceeded smoothly in the presence of a catalytic amount of Et3N·HI together with a palladium catalyst and exhibited great functional group tolerance. The employment of Et3N·HI not only avoids the prior preparation of alkyl halide substrates but also is the key to the high chemoselective ring-opening of epoxides. Preliminary mechanism explorations strongly support a radical pathway, and the catalytic cycle was established tentatively according to the mechanism investigation experiments.
中文翻译:

Pd/Et3N·HI催化分子内C-H烷基化通过环氧化物的高区域选择性开环获得[a]-环状吲哚
实现了 Pd/Et 3 N·HI 催化的吲哚与环氧化物的分子内 C-H 烷基化,以提供带有醇基的N-稠合吲哚骨架(5-、6-和 7-元环)。在催化量的Et 3 N·HI 和钯催化剂的存在下,转化过程顺利进行,并表现出很好的官能团耐受性。Et 3 N·HI的使用不仅避免了卤代烷底物的前期制备,而且是环氧化物高化学选择性开环的关键。初步机理探索强烈支持自由基途径,根据机理研究实验初步建立了催化循环。
更新日期:2022-06-07
中文翻译:

Pd/Et3N·HI催化分子内C-H烷基化通过环氧化物的高区域选择性开环获得[a]-环状吲哚
实现了 Pd/Et 3 N·HI 催化的吲哚与环氧化物的分子内 C-H 烷基化,以提供带有醇基的N-稠合吲哚骨架(5-、6-和 7-元环)。在催化量的Et 3 N·HI 和钯催化剂的存在下,转化过程顺利进行,并表现出很好的官能团耐受性。Et 3 N·HI的使用不仅避免了卤代烷底物的前期制备,而且是环氧化物高化学选择性开环的关键。初步机理探索强烈支持自由基途径,根据机理研究实验初步建立了催化循环。

































 京公网安备 11010802027423号
京公网安备 11010802027423号