当前位置:
X-MOL 学术
›
Appl. Organomet. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pd(II)–PPh3 complexes of halogen substituted acylthiourea ligands: Biomolecular interactions and in vitro anti-proliferative activity
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2022-05-30 , DOI: 10.1002/aoc.6765
Dorothy Priyanka Dorairaj, Jebiti Haribabu, Yu-Lun Chang, Cesar Echeverria, Sodio C. N. Hsu, Ramasamy Karvembu
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2022-05-30 , DOI: 10.1002/aoc.6765
Dorothy Priyanka Dorairaj, Jebiti Haribabu, Yu-Lun Chang, Cesar Echeverria, Sodio C. N. Hsu, Ramasamy Karvembu
|
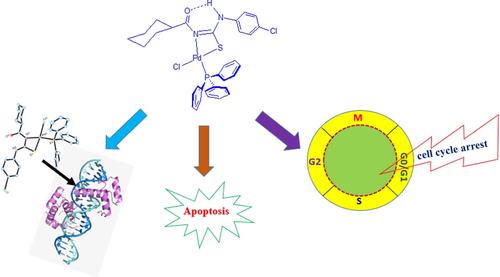
Herein, we report the synthesis of four new Pd(II) acylthiourea complexes (C1–C4) to study the effect of halogen substituted acylthiourea ligands on the biological applications of their Pd(II)–PPh3 complexes. The complexes were characterized by various spectroscopic and analytical methods. The distorted square planar geometry of complexes was confirmed by single crystal X-ray diffraction study, which also revealed the bidentate (N, S) coordination of the ligands with Pd(II). Interactions of the complexes with biomolecules (DNA/BSA) were investigated by spectroscopic and docking tools. All the complexes showed good binding ability with the targeted biomolecules. Further, in vitro anti-proliferative activity of the complexes was investigated by performing MTT assay on cancer (HeLa-cervical, HCT116-colorectal, and HepG2-hepatic) and normal (HEK293-embryonic kidney) cell lines. All the complexes displayed remarkable activity on the three cancer cells, and among them, complex C2 bearing chlorine substituted acylthiourea showed superior activity on HeLa cells with an IC50 value of 6.5 μM, which was higher than that of cisplatin. Apoptosis induced by C2 on HeLa cells was assessed by AO/EB, DAPI and DNA laddering assays, and flow cytometry. Finally, the apoptotic efficiency of C2 was tested by cell cycle analysis, wherein the complex induced the cell cycle arrest in HeLa cells at G0/G1 phase.
中文翻译:

卤素取代的酰基硫脲配体的 Pd(II)-PPh3 配合物:生物分子相互作用和体外抗增殖活性
在此,我们报告了四种新型 Pd(II) 酰基硫脲配合物 ( C1 – C4 ) 的合成,以研究卤素取代的酰基硫脲配体对其 Pd(II)–PPh 3配合物的生物学应用的影响。通过各种光谱和分析方法对配合物进行表征。通过单晶 X 射线衍射研究证实了配合物的扭曲方形平面几何形状,该研究还揭示了配体与 Pd(II) 的二齿 (N, S) 配位。通过光谱和对接工具研究了配合物与生物分子 (DNA/BSA) 的相互作用。所有复合物均显示出与靶向生物分子良好的结合能力。此外,体外通过对癌症(HeLa-宫颈、HCT116-结肠直肠和 HepG2-肝)和正常(HEK293-胚胎肾)细胞系进行 MTT 测定来研究复合物的抗增殖活性。所有配合物对三种癌细胞均表现出显着的活性,其中,氯代酰基硫脲配合物C2对HeLa细胞具有优异的活性,IC 50值为6.5 μM,高于顺铂。通过 AO/EB、DAPI 和 DNA 梯形分析以及流式细胞术评估C2在 HeLa 细胞上诱导的细胞凋亡。最后,通过细胞周期分析测试了C2的凋亡效率,其中复合物诱导HeLa细胞的细胞周期停滞在G0/G1期。
更新日期:2022-05-30
中文翻译:

卤素取代的酰基硫脲配体的 Pd(II)-PPh3 配合物:生物分子相互作用和体外抗增殖活性
在此,我们报告了四种新型 Pd(II) 酰基硫脲配合物 ( C1 – C4 ) 的合成,以研究卤素取代的酰基硫脲配体对其 Pd(II)–PPh 3配合物的生物学应用的影响。通过各种光谱和分析方法对配合物进行表征。通过单晶 X 射线衍射研究证实了配合物的扭曲方形平面几何形状,该研究还揭示了配体与 Pd(II) 的二齿 (N, S) 配位。通过光谱和对接工具研究了配合物与生物分子 (DNA/BSA) 的相互作用。所有复合物均显示出与靶向生物分子良好的结合能力。此外,体外通过对癌症(HeLa-宫颈、HCT116-结肠直肠和 HepG2-肝)和正常(HEK293-胚胎肾)细胞系进行 MTT 测定来研究复合物的抗增殖活性。所有配合物对三种癌细胞均表现出显着的活性,其中,氯代酰基硫脲配合物C2对HeLa细胞具有优异的活性,IC 50值为6.5 μM,高于顺铂。通过 AO/EB、DAPI 和 DNA 梯形分析以及流式细胞术评估C2在 HeLa 细胞上诱导的细胞凋亡。最后,通过细胞周期分析测试了C2的凋亡效率,其中复合物诱导HeLa细胞的细胞周期停滞在G0/G1期。





































 京公网安备 11010802027423号
京公网安备 11010802027423号