Insect Conservation and Diversity ( IF 3.2 ) Pub Date : 2022-06-01 , DOI: 10.1111/icad.12589 Andrew H. Aldercotte 1 , Dylan T. Simpson 1 , Rachael Winfree 2
|
INTRODUCTION
There is widespread concern about declines in bees and the pollination services they provide (Cameron & Sadd, 2020; Goulson et al., 2015). Bees are important pollinators in both agricultural and natural ecosystems (Ollerton, 2017; Willmer et al., 2017), and bee population declines could adversely affect food production, as well as the reproductive success and future composition of natural plant communities (Sargent & Ackerly, 2008; van der Sluijs & Vaage, 2016). Yet, there have been surprisingly few long-term studies of bee populations that used standardised methods to measure changes in bee abundance over time and thus are able to detect decline in its most basic sense: a decrease in the number of individuals over time.
In contrast, most studies on bee decline have used data compiled from a wide range of sources, such as citizen science projects and museum collections, that were gathered under different protocols and with non-standardised or unquantified sampling effort (Bartomeus et al., 2013; Biesmeijer et al., 2006; Carvalheiro et al., 2013; Powney et al., 2019; Senapathi et al., 2015; Soroye et al., 2020; Zattara & Aizen, 2021). In such datasets, when the number of individuals of a species decreases over time (i.e., its abundance declines), it can be difficult to know whether that is a because the species was indeed declining, or whether apparent decline is an artefact of the sampling design. First, when sampling effort is not standardised over time, a species can erroneously appear to decline because less effort was made to sample it (or its habitat) in the recent period. Second, a rarer species can appear to decline because early collectors targeted rare species, whereas later collectors tended to use passive collection methods that sample species in proportion to their abundance and thus collect mainly common species (Gotelli et al., 2021). Third, a species can appear to decline if other species in the same dataset increased over time and therefore constitute a larger proportion of the more recent samples (Bartomeus & Winfree, 2013). Although calibration methods have been developed that can make museum and field-data comparable (Gotelli et al., 2021), this does not negate the importance of having local scale time series for which sampling effort is standardised over time, and thus declines in abundance can be detected directly (Goulson et al., 2015).
The two longest-term studies that did directly measure changes in abundance over time in bee communities were conducted in Panama and Spain and found that populations were predominantly stable or increasing over time (Herrera, 2019; Roubik, 2001; Roubik et al., 2021; Roubik & Wolda, 2000). However, both studies were also conducted in relatively undisturbed natural habitats and thus might under-estimate bee declines in areas with greater human impacts. For North America, specifically, the few published time-series studies of bee populations show conflicting results. Standardised studies based on observations or netting at flowers have been done only for the genus Bombus (the bumble bees) and only in natural systems. In Colorado, USA, peak annual abundance for three bumble bee species showed no significant change over 8 years (2009–2016) (Ogilvie et al., 2017), while in California, USA, a bumble bee species complex showed a roughly 10-fold decline in the number of bees counted per unit of survey area over 15 years from 1999 to 2014 (Thomson, 2016). Two additional North American time-series studies used pan traps to monitor bee populations over time. Pan traps have the disadvantage of being biased towards catching Halictids and some other small-bodied taxa (Portman et al., 2020) but have the advantage of providing large sample sizes (Westphal et al., 2008). Onuferko et al. (2018) found a significant decline in aggregate bee abundance in Ontario, Canada from 2003 to 2013, although the magnitude of the decline was not reported. Graham et al. found a dramatic decline in aggregate bee abundance (to 39% of initial abundance) and then partial recovery (to 70% of initial abundance) between 2004 and 2018 at highbush blueberry (Vaccinium corymbosum L.) farms in Michigan (Graham et al., 2021). Other published time-series studies of bee abundance were either based on short time series (<5 years) or did not report on the statistical significance of changes in total abundances over time (Cane & Payne, 1993; Franzen & Nilsson, 2013; Herrera, 1988; Iserbyt & Rasmont, 2012; Owen, 1978).
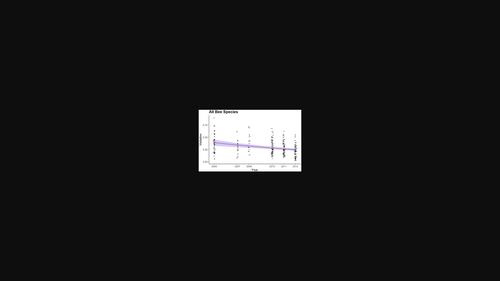
Even when time series are long and sample sizes are large, high inter-annual variation in bee abundance presents a challenge to the detection of trends (Cane & Tepedino, 2001; Didham et al., 2020; Williams et al., 2001). Bee abundance at both the species and the community levels can vary by an order of magnitude between years, in both agricultural (Cane & Payne, 1993; Senapathi et al., 2021) and natural ecosystems (Herrera, 1988; Ogilvie et al., 2017; Roubik, 2001). The drivers of this variation are not well determined but may include variation in annual weather patterns (Kammerer et al., 2021; Ogilvie et al., 2017), infection/infestation rates of brood parasites and pathogens, especially fungal pathogens (Danforth et al., 2019), pesticide application (e.g. Mallinger et al., 2015), and changing landscapes and land management practises (e.g. Humbert et al., 2012; Kennedy et al., 2013; Lichtenberg et al., 2017). This stochastic temporal variation in abundance reduces the power of regression-based models to identify trends (Gerrodette, 1987), or, alternatively, increases the risk of erroneously identifying a trend where there is none. For example, given a system with high interannual variation but no long-term trend, analysis of short time series can find significant positive or negative trends, particularly when bounded by high and low years (Figure 1, see Didham et al., 2020 for a graphical representation of the many challenges associated with trend analysis in insect populations). Said another way, interannual variation (or process error) increases both type I and type II error rates because it inherently violates the regression model's null hypothesis that abundances are the same across time (Gerrodette, 1987). As a result, studies seeking to detect bee population declines require higher-than-usual sampling intensity over longer periods in order to differentiate long-term trends in mean abundance from changes attributable to stochastic inter-annual variation (Gibbs et al., 1998; White, 2019). Approaches that incorporate process error into their expectations, such as state space models (Clark & Bjornstad, 2004; Humbert et al., 2009), power analysis via simulation (Gerrodette, 1987), or permutational null models can be helpful in differentiating actual trends from noise (Fox et al., 2019).

Here, we evaluate 6 years of data (collected over 8 years, 2005–2012) on bee abundance at watermelon flowers (Citrullus lanatus Thunb.) at 19 farms in the Mid-Atlantic USA. Because our study design standardises plant species and floral density across years, it controls for variation in bee abundance attributable to these factors. Furthermore, by working in an agricultural system, we directly measure bee declines as they are affecting crop pollination. We ask (i) whether there were trends in bee abundance at crop flowers over time and (ii) whether trends in abundance differed among bee taxa. In addition, we use data we collected on the per visit pollen deposition of bees to evaluate the strength of the relationship between total visitation by bees and the pollination services they provide.
中文翻译:

野生蜜蜂的作物访问量在 8 年的时间序列中下降:一个显着的趋势,还是只是显着的年际变化?
介绍
人们普遍担心蜜蜂的减少及其提供的授粉服务(Cameron & Sadd, 2020 年;Goulson 等人, 2015 年)。蜜蜂是农业和自然生态系统中重要的传粉媒介(Ollerton, 2017 年;Willmer 等人, 2017 年),蜜蜂数量下降可能对粮食生产以及自然植物群落的繁殖成功和未来组成产生不利影响(Sargent 和 Ackerly , 2008 年;范德斯鲁伊斯和 Vaage, 2016 年)。然而,令人惊讶的是,很少有关于蜜蜂种群的长期研究使用标准化方法来测量蜜蜂数量随时间的变化,因此能够检测到最基本意义上的下降:随着时间的推移个体数量的减少。
相比之下,大多数关于蜜蜂衰退的研究都使用了从广泛来源收集的数据,例如公民科学项目和博物馆藏品,这些数据是根据不同的协议和非标准化或非量化的抽样工作收集的(Bartomeus 等, 2013;Biesmeijer 等人, 2006 年;Carvalheiro 等人, 2013 年;Powney 等人, 2019 年;Senapathi 等人, 2015 年;Soroye 等人, 2020 年;Zattara 和 Aizen, 2021 年)。在这样的数据集中,当一个物种的个体数量随着时间的推移而减少(即其丰度下降)时,很难知道这是否是因为该物种确实在下降,或者明显下降是否是抽样的假象设计。首先,当采样工作随着时间的推移没有标准化时,一个物种可能会错误地出现衰退,因为最近一段时间对其(或其栖息地)的采样工作较少。其次,稀有物种可能会出现下降,因为早期的采集者针对的是稀有物种,而后来的采集者倾向于使用被动采集方法,根据物种的丰度对其进行采样,从而主要采集常见物种(Gotelli et al., 2021)。第三,如果同一数据集中的其他物种随着时间的推移而增加,一个物种可能会出现衰退,因此在最近的样本中占更大比例(Bartomeus & Winfree,2013)。尽管已经开发出可以使博物馆和现场数据具有可比性的校准方法(Gotelli 等人,2021 年),但这并没有否定具有局部尺度时间序列的重要性,该时间序列的采样工作随着时间的推移而标准化,因此丰度下降可以直接检测到 (Goulson et al., 2015 )。
在巴拿马和西班牙进行了两项直接测量蜜蜂群落丰度随时间变化的长期研究,发现随着时间的推移,种群数量主要是稳定的或增加的(Herrera,2019;Roubik,2001;Roubik 等人,2021;鲁比克和沃尔达,2000 年)。然而,这两项研究也是在相对不受干扰的自然栖息地进行的,因此可能低估了人类影响更大的地区蜜蜂的减少。具体而言,对于北美,少数已发表的蜜蜂种群时间序列研究显示出相互矛盾的结果。仅对熊蜂属进行了基于观察或花网的标准化研究(大黄蜂)并且仅在自然系统中。在美国科罗拉多州,三种大黄蜂物种的年丰度峰值在 8 年(2009-2016 年)中没有显着变化(Ogilvie 等人,2017 年),而在美国加利福尼亚州,大黄蜂物种复合体显示出大约 10-从 1999 年到 2014 年,每单位调查面积的蜜蜂数量在 15 年间下降了几倍(汤姆森,2016 年)。另外两项北美时间序列研究使用平底锅来监测蜜蜂种群随时间的变化。Pan trap 的缺点是偏向于捕捉 Halictids 和其他一些小体型分类群(Portman 等人,2020 年),但具有提供大样本量的优势(Westphal 等人,2008 年)。Onuferko 等人。( 2018) 发现从 2003 年到 2013 年,加拿大安大略省的蜜蜂总丰度显着下降,尽管没有报告下降的幅度。格雷厄姆等人。发现在 2004 年至 2018 年期间,密歇根州的高丛蓝莓 ( Vaccinium corymbosum L.) 农场的蜜蜂总丰度急剧下降(至初始丰度的 39%),然后部分恢复(至初始丰度的 70%)(Graham 等人,2021 年)。其他已发表的蜜蜂丰度时间序列研究要么基于短时间序列(<5 年),要么没有报告总丰度随时间变化的统计显着性(Cane & Payne, 1993 ; Franzen & Nilsson, 2013 ; Herrera , 1988 ; 伊塞尔比特和拉斯蒙特, 2012; 欧文,1978 年)。
即使时间序列很长且样本量很大,蜜蜂丰度的高年际变化也对趋势检测提出了挑战(Cane & Tepedino,2001;Didham 等,2020;Williams 等,2001)。在农业 (Cane & Payne, 1993 ; Senapathi et al., 2021 ) 和自然生态系统 (Herrera, 1988 ; Ogilvie et al., 2017 年;鲁比克,2001 年)。这种变化的驱动因素尚未确定,但可能包括年度天气模式的变化(Kammerer 等人,2021; Ogilvie 等人,2017 年)、育雏寄生虫和病原体的感染/侵染率,尤其是真菌病原体(Danforth 等人,2019 年)、杀虫剂应用(例如 Mallinger 等人,2015 年),以及不断变化的景观和土地管理实践(例如 Humbert 等人,2012 年;Kennedy 等人,2013 年;Lichtenberg 等人,2017 年)。这种丰度的随机时间变化降低了基于回归的模型识别趋势的能力(Gerrodette,1987),或者,在没有趋势的情况下,会增加错误识别趋势的风险。例如,给定一个具有高年际变化但没有长期趋势的系统,对短时间序列的分析可以发现显着的正或负趋势,特别是在高年和低年的情况下(图 1,参见 Didham 等人,2020与昆虫种群趋势分析相关的许多挑战的图形表示)。换句话说,年际变化(或过程错误)会增加 I 型和 II 型错误率,因为它本质上违反了回归模型的零假设,即丰度在时间上是相同的(Gerrodette,1987)。因此,旨在检测蜜蜂数量减少的研究需要在较长时期内高于通常的采样强度,以便区分平均丰度的长期趋势和可归因于随机年际变化的变化(Gibbs 等,1998;怀特,2019 年)。将过程误差纳入其预期的方法,例如状态空间模型 (Clark & Bjornstad, 2004 ; Humbert et al., 2009 )、通过模拟进行的功率分析 (Gerrodette, 1987 ) 或置换零模型有助于区分实际趋势来自噪音(Fox 等人,2019 年)。

在这里,我们评估了美国中大西洋 19 个农场的6 年数据(2005-2012 年 8 年收集)关于西瓜花(Citrullus lanatus Thunb.)蜜蜂丰度的数据。因为我们的研究设计标准化了植物种类和多年的花卉密度,所以它控制了由这些因素引起的蜜蜂丰度的变化。此外,通过在农业系统中工作,我们直接测量蜜蜂数量减少,因为它们影响作物授粉。我们询问(i)随着时间的推移,作物花朵中的蜜蜂丰度是否存在趋势,以及(ii)蜜蜂分类群之间的丰度趋势是否不同。此外,我们使用我们收集的关于蜜蜂每次访问花粉沉积的数据来评估蜜蜂总访问次数与它们提供的授粉服务之间关系的强度。

































 京公网安备 11010802027423号
京公网安备 11010802027423号