当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cobalt–Carbon Bonding in a Salen-Supported Cobalt(IV) Alkyl Complex Postulated in Oxidative MHAT Catalysis
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-06-03 , DOI: 10.1021/jacs.2c02128 Conner V Wilson 1 , Dongyoung Kim 1 , Ajay Sharma 2 , Reagan X Hooper 1 , Rinaldo Poli 3 , Brian M Hoffman 2 , Patrick L Holland 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-06-03 , DOI: 10.1021/jacs.2c02128 Conner V Wilson 1 , Dongyoung Kim 1 , Ajay Sharma 2 , Reagan X Hooper 1 , Rinaldo Poli 3 , Brian M Hoffman 2 , Patrick L Holland 1
Affiliation
|
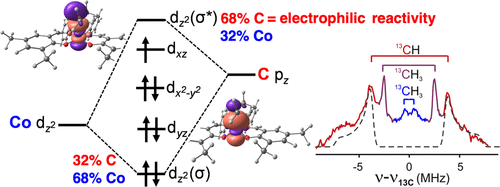
The catalytic hydrofunctionalization of alkenes through radical-polar crossover metal hydrogen atom transfer (MHAT) offers a mild pathway for the introduction of functional groups in sterically congested environments. For M = Co, this reaction is often proposed to proceed through secondary alkylcobalt(IV) intermediates, which have not been characterized unambiguously. Here, we characterize a metastable (salen)Co(isopropyl) cation, which is capable of forming C–O bonds with alcohols as proposed in the catalytic reaction. Electron nuclear double resonance (ENDOR) spectroscopy of this formally cobalt(IV) species establishes the presence of the cobalt–carbon bond, and accompanying DFT calculations indicate that the unpaired electron is localized on the cobalt center. Both experimental and computational studies show that the cobalt(IV)–carbon bond is stronger than the analogous bond in its cobalt(III) analogue, which is opposite of the usual oxidation state trend of bond energies. This phenomenon is attributable to an inverted ligand field that gives the bond Coδ−–Cδ+ character and explains its electrophilic reactivity at the alkyl group. The inverted Co–C bond polarity also stabilizes the formally cobalt(IV) alkyl complex so that it is accessible at unusually low potentials. Even another cobalt(III) complex, [(salen)CoIII]+, is capable of oxidizing (salen)CoIII(iPr) to the formally cobalt(IV) state. These results give insight into the electronic structure, energetics, and reactivity of a key reactive intermediate in oxidative MHAT catalysis.
中文翻译:

Salen 支持的钴 (IV) 烷基络合物中的钴碳键合假设在氧化 MHAT 催化中
通过自由基-极性交叉金属氢原子转移 (MHAT) 对烯烃进行催化加氢官能化,为在空间拥挤的环境中引入官能团提供了一条温和的途径。对于 M = Co,通常建议该反应通过仲烷基钴 (IV) 中间体进行,但尚未明确表征。在这里,我们表征了亚稳态 (salen)Co(isopropyl) 阳离子,它能够按照催化反应中的建议与醇形成 C-O 键。这种正式钴 (IV) 物种的电子核双共振 (ENDOR) 光谱确定了钴-碳键的存在,并且伴随的 DFT 计算表明未配对的电子位于钴中心。实验和计算研究均表明,钴 (IV)-碳键比其钴 (III) 类似物中的类似键更强,这与键能的通常氧化态趋势相反。这种现象可归因于一个反转的配体场,它给出了 Co 键δ− –C δ+特征并解释其在烷基上的亲电反应性。反向的 Co-C 键极性也稳定了形式上的钴 (IV) 烷基络合物,因此它可以在异常低的电位下获得。甚至另一种钴 (III) 配合物 [(salen)Co III ] +也能够将 (salen)Co III ( i Pr) 氧化为形式上的钴 (IV) 态。这些结果有助于深入了解氧化 MHAT 催化中关键反应中间体的电子结构、能量学和反应性。
更新日期:2022-06-03
中文翻译:

Salen 支持的钴 (IV) 烷基络合物中的钴碳键合假设在氧化 MHAT 催化中
通过自由基-极性交叉金属氢原子转移 (MHAT) 对烯烃进行催化加氢官能化,为在空间拥挤的环境中引入官能团提供了一条温和的途径。对于 M = Co,通常建议该反应通过仲烷基钴 (IV) 中间体进行,但尚未明确表征。在这里,我们表征了亚稳态 (salen)Co(isopropyl) 阳离子,它能够按照催化反应中的建议与醇形成 C-O 键。这种正式钴 (IV) 物种的电子核双共振 (ENDOR) 光谱确定了钴-碳键的存在,并且伴随的 DFT 计算表明未配对的电子位于钴中心。实验和计算研究均表明,钴 (IV)-碳键比其钴 (III) 类似物中的类似键更强,这与键能的通常氧化态趋势相反。这种现象可归因于一个反转的配体场,它给出了 Co 键δ− –C δ+特征并解释其在烷基上的亲电反应性。反向的 Co-C 键极性也稳定了形式上的钴 (IV) 烷基络合物,因此它可以在异常低的电位下获得。甚至另一种钴 (III) 配合物 [(salen)Co III ] +也能够将 (salen)Co III ( i Pr) 氧化为形式上的钴 (IV) 态。这些结果有助于深入了解氧化 MHAT 催化中关键反应中间体的电子结构、能量学和反应性。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号