当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stimulating the Pre-Catalyst Redox Reaction and the Proton–Electron Transfer Process of Cobalt Phthalocyanine for CO2 Electroreduction
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2022-06-03 , DOI: 10.1021/acs.jpcc.2c01125
Hengyu Li 1 , Jie Wei 1 , Xuya Zhu 1 , Lin Gan 1 , Tao Cheng 2 , Jia Li 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2022-06-03 , DOI: 10.1021/acs.jpcc.2c01125
Hengyu Li 1 , Jie Wei 1 , Xuya Zhu 1 , Lin Gan 1 , Tao Cheng 2 , Jia Li 1
Affiliation
|
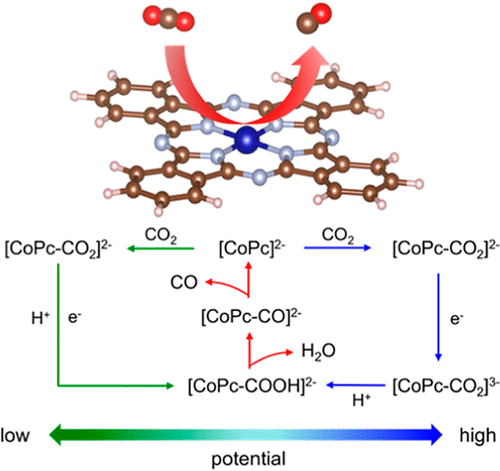
The mechanism for electrochemical carbon dioxide reduction reaction (CO2RR) to carbon monoxide on cobalt phthalocyanine (CoPc) in aqueous electrolytes remains debatable, impeding the design of high-performance catalysts. By using a quasi-empirical protocol with density functional theory calculations, we identify the mechanisms of two important steps for CO2RR on CoPc: the reduction of CoIIPc to form catalytically active [CoIIPc]2– for CO2 adsorption and the proton–electron transfer to the key intermediate [CoPc-COO]2– to form [CoPc-COOH]2–. According to the charge states and pKa analysis, the formation of the adsorbed carboxyl (*COOH) takes place via the concerted proton–electron transfer process at low potentials, and the sequential proton–electron transfer process becomes thermodynamically favored at more reductive potentials, which successfully elucidates the potential-dependent reaction kinetics of CO2RR on CoPc catalysts. Electron-withdrawing substituents of CoPc would enhance the reduction of CoPc but hinder the protonation of *CO2, which accounts for previous conflicting results. Our findings not only deepen the understanding of CoPc-catalyzed CO2RR but also provide a guideline for molecular engineering of CoPc-based catalysts.
中文翻译:

激发钴酞菁的催化剂前氧化还原反应和质子-电子转移过程用于 CO2 电还原
在水性电解质中钴酞菁 (CoPc) 上电化学二氧化碳还原反应 (CO 2 RR) 生成一氧化碳的机理仍有争议,这阻碍了高性能催化剂的设计。通过使用具有密度泛函理论计算的准经验协议,我们确定了 CO 2 RR 在 CoPc 上的两个重要步骤的机制:Co II Pc 还原形成催化活性 [Co II Pc] 2–用于 CO 2吸附和质子-电子转移到关键中间体 [CoPc-COO] 2-形成 [CoPc-COOH] 2-。根据电荷态和 pK a分析表明,吸附的羧基 (*COOH) 的形成是在低电位下通过协同的质子-电子转移过程发生的,并且在更高的还原电位下,连续的质子-电子转移过程在热力学上变得更有利,这成功地阐明了电位依赖性反应CoPc催化剂上CO 2 RR的动力学。CoPc 的吸电子取代基会增强 CoPc 的还原,但会阻碍 *CO 2的质子化,这是以前相互矛盾的结果的原因。我们的研究结果不仅加深了对 CoPc 催化的 CO 2 RR 的理解,而且为 CoPc 基催化剂的分子工程提供了指导。
更新日期:2022-06-03
中文翻译:

激发钴酞菁的催化剂前氧化还原反应和质子-电子转移过程用于 CO2 电还原
在水性电解质中钴酞菁 (CoPc) 上电化学二氧化碳还原反应 (CO 2 RR) 生成一氧化碳的机理仍有争议,这阻碍了高性能催化剂的设计。通过使用具有密度泛函理论计算的准经验协议,我们确定了 CO 2 RR 在 CoPc 上的两个重要步骤的机制:Co II Pc 还原形成催化活性 [Co II Pc] 2–用于 CO 2吸附和质子-电子转移到关键中间体 [CoPc-COO] 2-形成 [CoPc-COOH] 2-。根据电荷态和 pK a分析表明,吸附的羧基 (*COOH) 的形成是在低电位下通过协同的质子-电子转移过程发生的,并且在更高的还原电位下,连续的质子-电子转移过程在热力学上变得更有利,这成功地阐明了电位依赖性反应CoPc催化剂上CO 2 RR的动力学。CoPc 的吸电子取代基会增强 CoPc 的还原,但会阻碍 *CO 2的质子化,这是以前相互矛盾的结果的原因。我们的研究结果不仅加深了对 CoPc 催化的 CO 2 RR 的理解,而且为 CoPc 基催化剂的分子工程提供了指导。

































 京公网安备 11010802027423号
京公网安备 11010802027423号