Chinese Journal of Chemical Engineering Pub Date : 2022-06-01 , DOI: 10.1016/j.cjche.2022.05.007 Qiaoqiao Liu , Guihong Lin , Jian Zhou , Liangliang Huang , Chang Liu
|
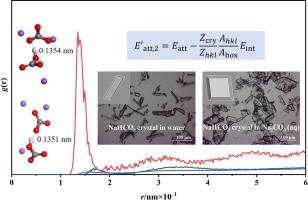
Adding Na2CO3 to the NaHCO3 cooling crystallizer, using the common ion effect to promote crystallization and improve product morphology, is a new process recently proposed in the literature. However, the mechanism of the impact of Na2CO3 on the crystal morphology is still indeterminate. In this work, the crystallization of NaHCO3 in water and Na2CO3–NaHCO3 aqueous solution was investigated by experiments and molecular dynamics simulations (MD). The crystallization results demonstrate that the morphology of NaHCO3 crystal changed gradually from needle-like to flake structure with the addition of Na2CO3. The simulation results indicate that the layer docking model and the modified attachment energy formula without considering the roughness of crystal surface can obtain the crystal morphology in agreement with the experimental results, but the lower molecules of the crystal layer have to be fixed during MD. Thermodynamic calculation of the NaHCO3 crystallization process verifies that the common ion effect from Na+ and the ionization equilibrium transformation from CO32– jointly promote the precipitation of NaHCO3 crystal. The radial distribution function analysis indicates that the oxygen atoms of Na2CO3 formed strong hydrogen bonds with the hydrogen atoms of the (0 1 1) face, which weakened the hydration of water molecules at the crystal surface, resulting in a significant change in the attachment energy of this crystal surface. In addition, Na+ and CO32– are more likely to accumulate on the (0 1 1) face, resulting in the fastest growth rate on this crystal surface, which eventually leads to a change in crystal morphology from needle-like to flake-like.
中文翻译:

NaHCO3–Na2CO3 水溶液中的氢键介导和浓度依赖性 NaHCO3 晶体形态:实验和计算机模拟
在NaHCO 3冷却结晶器中加入Na 2 CO 3,利用共离子效应促进结晶,改善产物形貌,是最近文献提出的一种新工艺。然而,Na 2 CO 3对晶体形态的影响机制仍未确定。在这项工作中,通过实验和分子动力学模拟 (MD) 研究了 NaHCO 3在水和 Na 2 CO 3 –NaHCO 3水溶液中的结晶。结晶结果表明NaHCO 3的形貌随着Na 2 CO 3的加入,晶体逐渐由针状结构转变为片状结构。模拟结果表明,不考虑晶体表面粗糙度的层对接模型和修改后的附着能公式可以获得与实验结果一致的晶体形貌,但晶体层的下层分子在MD过程中必须固定。NaHCO 3结晶过程的热力学计算验证了N a +的共离子效应和CO 3 2–的电离平衡转变共同促进了NaHCO 3的析出水晶。径向分布函数分析表明,Na 2 CO 3的氧原子与(0 1 1)面的氢原子形成强氢键,削弱了晶面水分子的水合作用,导致晶面的显着变化该晶体表面的附着能。此外,Na +和CO 3 2–更容易聚集在(0 1 1)面,导致该晶面上的生长速度最快,最终导致晶体形态从针状变为片状-喜欢。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号