当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
(Salen)Mn(III)-Catalyzed Enantioselective Intramolecular Haloamination of Alkenes through Chiral Aziridinium Ion Ring-Opening Sequence
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-06-01 , DOI: 10.1021/acscatal.2c02223
Hui Sun 1 , Huijian Shang 1 , Bin Cui 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-06-01 , DOI: 10.1021/acscatal.2c02223
Hui Sun 1 , Huijian Shang 1 , Bin Cui 1
Affiliation
|
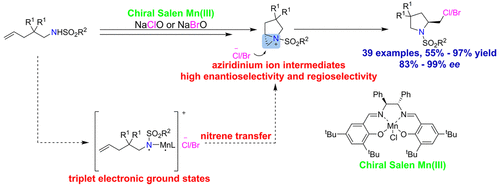
Asymmetric catalysis with a chiral (Salen)Mn(III) complex is applied successfully to highly enantioselective intramolecular haloamination reactions of alkenes through a chiral aziridinium ion ring-opening sequence. Computational and experimental studies suggested that the C5–C6 bond polarization has a major effect on the regioselectivity of the chiral aziridines ring intermediate, and steric hindrance of groups at C5 or C6 plays a minor role. Various amino-alkenes can cyclize under the reaction conditions to obtain 2-brominated/chlorinated pyrrolidine and indoline derivatives with excellent yield and enantioselectivity.
中文翻译:

(Salen)Mn(III)-通过手性氮丙啶离子开环序列催化烯烃的对映选择性分子内卤化
手性 (Salen)Mn(III) 配合物的不对称催化通过手性氮丙啶离子开环序列成功应用于烯烃的高对映选择性分子内卤化反应。计算和实验研究表明,C5-C6 键极化对手性氮丙啶环中间体的区域选择性有主要影响,C5 或 C6 基团的空间位阻起次要作用。多种氨基烯烃在该反应条件下可以环化得到2-溴代/氯代吡咯烷和二氢吲哚衍生物,具有优异的收率和对映选择性。
更新日期:2022-06-01
中文翻译:

(Salen)Mn(III)-通过手性氮丙啶离子开环序列催化烯烃的对映选择性分子内卤化
手性 (Salen)Mn(III) 配合物的不对称催化通过手性氮丙啶离子开环序列成功应用于烯烃的高对映选择性分子内卤化反应。计算和实验研究表明,C5-C6 键极化对手性氮丙啶环中间体的区域选择性有主要影响,C5 或 C6 基团的空间位阻起次要作用。多种氨基烯烃在该反应条件下可以环化得到2-溴代/氯代吡咯烷和二氢吲哚衍生物,具有优异的收率和对映选择性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号