Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2022-05-30 , DOI: 10.1016/j.molstruc.2022.133411 Saad R. El-Zemity , Mohamed E.I. Badawy , Kareem E.E. Esmaiel , Mai M. Badr
|
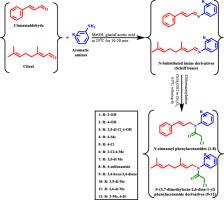
A series of N-cinnamyl phenylacetamides (1-8) and N-(3,7-dimethylocta-2,6-dien-1-yl) phenylacetamide derivatives (9-12) with different active moieties have been designed, synthesized and tested for antioxidant and antimicrobial activity. The synthetic protocol was based on the formation of Schiff bases followed by chloroacetylation of imines. The chemical structures of the compounds were recognized by 1H-NMR, 13C-NMR, and MS spectral techniques. The structure and configuration of the substituents are shown to significantly influence the antioxidant and antimicrobial activity of the compounds under study. Compounds 1-4 and 6-10 exhibited superior antioxidant activity (IC50 = 9.92-19.06 µM) compared to α-tocopherol (IC50 = 26 µM). N-(3,7-dimethylocta-2,6-dien-1-yl) phenylacetamide derivatives (9-12) showed excellent antibacterial activities against all the tested strains with MIC values in the range of 10-230 µM comparable to those of amoxicillin (80-275 µM) against Bacillus cereus (G+), Staphylococcus aureus (G+), Escherichia coli (G-), and Pseudomonas aeruginosa (G-). Besides, the compounds also exhibited candidacidal activity against Candida albicans and (E)-2-chloro-N-(3,7-dimethylocta-2,6-dien-1-yl)-N-(2-ethyl-6-methylphenyl)acetamide 12 was the most active compound (EC50 = 423.62 µM). Molecular docking, drug-likeness data, physicochemical properties, and ADMET (absorption, distribution, metabolism, excretion, and toxicity) parameters of the compounds were in silico computed. The derivatives presented good properties for Lipinski's parameters, poor solubility in the aqueous medium (Log S of -4.06 to -5.97), and PSA <140, indicating good permeability in biological membranes and gastrointestinal absorption. Molecular docking to the active sites of NADPH oxidase (PDB: 1W6X), penicillin-binding protein 2a (PDB: 1VQQ), and lanosterol 14-alpha demethylase (PDB 1EA1) revealed that most compounds displayed minimal binding energy and have a good affinity toward the active pocket of each enzyme. This is the first article to describe the antioxidant and antimicrobial properties of N-cinnamyl phenylacetamide and N-(3,7-dimethylocta-2,6-dien-1-yl) phenylacetamide derivatives.
中文翻译:

一些 N-肉桂基苯乙酰胺和 N-(3,7-dimethylocta-2,6-dien-1-yl) 苯乙酰胺衍生物的合成、抗氧化、抗菌和分子对接研究
设计、合成和测试了一系列具有不同活性部分的N-肉桂基苯乙酰胺 ( 1-8 ) 和N- (3,7-二甲基八-2,6-二烯-1-基) 苯乙酰胺衍生物 ( 9-12 )用于抗氧化和抗菌活性。合成方案基于席夫碱的形成,然后是亚胺的氯乙酰化。化合物的化学结构通过1 H-NMR、13 C-NMR和MS光谱技术进行识别。取代基的结构和构型显示出显着影响所研究化合物的抗氧化和抗微生物活性。化合物1-4和6-10 与 α-生育酚 (IC 50 = 26 µM ) 相比,表现出优异的抗氧化活性 (IC 50 = 9.92-19.06 µM)。N-(3,7-dimethylocta-2,6-dien-1-yl) phenylacetamide 衍生物 ( 9-12 ) 对所有受试菌株显示出优异的抗菌活性,MIC 值在 10-230 µM 范围内,与阿莫西林 (80-275 µM) 针对蜡状芽孢杆菌(G+)、金黄色葡萄球菌(G+)、大肠杆菌(G-) 和铜绿假单胞菌(G-)。此外,这些化合物还表现出对白色念珠菌和 ( E )-2-氯-N的念珠菌活性。-(3,7-dimethylocta-2,6-dien-1-yl)- N -(2-ethyl-6-methylphenyl)acetamide 12是最活跃的化合物 (EC 50 = 423.62 µM)。化合物的分子对接、药物相似性数据、理化性质和 ADMET(吸收、分布、代谢、排泄和毒性)参数在计算机上计算。衍生物在 Lipinski 参数方面表现出良好的特性,在水性介质中的溶解度较差(Log S 为 -4.06 至 -5.97),PSA <140,表明在生物膜中具有良好的渗透性和胃肠道吸收。与 NADPH 氧化酶 (PDB: 1W6X)、青霉素结合蛋白 2a (PDB: 1VQQ) 和羊毛甾醇 14-α 去甲基化酶 (PDB 1EA1) 的活性位点的分子对接表明,大多数化合物显示出最小的结合能并且对每种酶的活性口袋。这是第一篇描述N-肉桂基苯乙酰胺和N- (3,7-dimethylocta-2,6-dien-1-yl) 苯乙酰胺衍生物的抗氧化和抗菌特性的文章。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号