当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mediating the Oxidizing Capability of Surface-Bound Hydroxyl Radicals Produced by Photoelectrochemical Water Oxidation to Convert Glycerol into Dihydroxyacetone
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-05-30 , DOI: 10.1021/acscatal.2c01319 Yang Liu 1 , Miao Wang 1 , Bing Zhang 2 , Dongpeng Yan 3 , Xu Xiang 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-05-30 , DOI: 10.1021/acscatal.2c01319 Yang Liu 1 , Miao Wang 1 , Bing Zhang 2 , Dongpeng Yan 3 , Xu Xiang 1
Affiliation
|
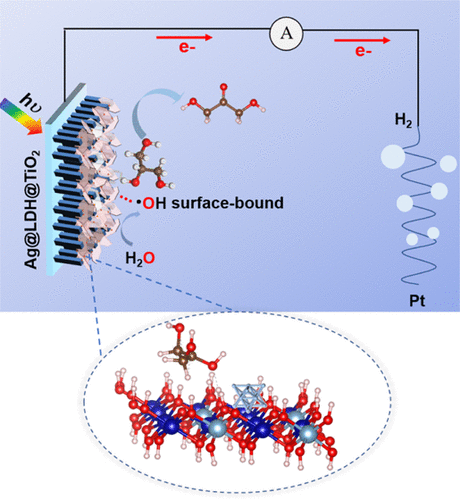
Highly selective oxidation of a single specific hydroxyl group in glycerol is attractive but challenging because glycerol contains three similar hydroxyl groups. In this work, we developed a ternary photoanode comprising Ag nanoparticle-supported layered double hydroxide (LDH) nanosheets on TiO2 (denoted Ag@LDH@TiO2) for the glycerol selective oxidation to 1,3-dihydroxyacetone via photoelectrochemical water oxidation under neutral conditions. It was proved that hydroxyl radicals generated by water oxidation were the dominating active oxygen species and oxygen atoms in the main oxidation product came from water. The LDHs and Ag nanoparticles enhanced the selectivity of secondary hydroxyl oxidation, and the Ag nanoparticles further accelerated the corresponding kinetics. The Ag@LDH@TiO2 photoanode exhibited a 1,3-dihydroxyacetone selectivity of 72% at 1.2 V vs reversible hydrogen electrode, which is obviously higher than that of pure TiO2 (23.5%) and surpasses most materials reported thus far. The role of LDHs and Ag nanoparticles in selective oxidation of glycerol was revealed through detailed spectroscopic and computational studies. Specifically, Fourier transform infrared spectroscopy analysis revealed that the middle hydroxyl group is preferentially adsorbed to LDH surfaces, while density function theory calculations verified that the surface-bound hydroxyl radicals mediated dehydrogenation barriers of middle carbon of adsorbed glycerol; the Ag nanoparticles promoted the selective adsorption of middle hydroxyl of glycerol, which further induced its selective oxidation.
中文翻译:

介导光电化学水氧化产生的表面结合羟基自由基的氧化能力以将甘油转化为二羟基丙酮
甘油中单个特定羟基的高选择性氧化具有吸引力但具有挑战性,因为甘油含有三个相似的羟基。在这项工作中,我们开发了一种三元光阳极,包括在 TiO 2上的 Ag 纳米颗粒负载的层状双氢氧化物 (LDH) 纳米片(表示为 Ag@LDH@TiO 2),用于在中性条件下通过光电化学水氧化将甘油选择性氧化为 1,3-二羟基丙酮。条件。证明水氧化产生的羟基自由基是主要的活性氧物种,主要氧化产物中的氧原子来自于水。LDHs和Ag纳米粒子增强了仲羟基氧化的选择性,Ag纳米粒子进一步加速了相应的动力学。Ag@LDH@TiO2光阳极在 1.2 V 时对可逆氢电极的 1,3-二羟基丙酮选择性为 72%,明显高于纯 TiO 2的选择性(23.5%),超过了迄今为止报道的大多数材料。通过详细的光谱和计算研究揭示了 LDH 和 Ag 纳米颗粒在甘油选择性氧化中的作用。具体而言,傅里叶变换红外光谱分析表明,中间羟基优先吸附在LDH表面,而密度泛函理论计算验证了表面结合的羟基自由基介导了吸附甘油中间碳的脱氢势垒;Ag纳米粒子促进了甘油中间羟基的选择性吸附,进一步诱导了甘油的选择性氧化。
更新日期:2022-05-30
中文翻译:

介导光电化学水氧化产生的表面结合羟基自由基的氧化能力以将甘油转化为二羟基丙酮
甘油中单个特定羟基的高选择性氧化具有吸引力但具有挑战性,因为甘油含有三个相似的羟基。在这项工作中,我们开发了一种三元光阳极,包括在 TiO 2上的 Ag 纳米颗粒负载的层状双氢氧化物 (LDH) 纳米片(表示为 Ag@LDH@TiO 2),用于在中性条件下通过光电化学水氧化将甘油选择性氧化为 1,3-二羟基丙酮。条件。证明水氧化产生的羟基自由基是主要的活性氧物种,主要氧化产物中的氧原子来自于水。LDHs和Ag纳米粒子增强了仲羟基氧化的选择性,Ag纳米粒子进一步加速了相应的动力学。Ag@LDH@TiO2光阳极在 1.2 V 时对可逆氢电极的 1,3-二羟基丙酮选择性为 72%,明显高于纯 TiO 2的选择性(23.5%),超过了迄今为止报道的大多数材料。通过详细的光谱和计算研究揭示了 LDH 和 Ag 纳米颗粒在甘油选择性氧化中的作用。具体而言,傅里叶变换红外光谱分析表明,中间羟基优先吸附在LDH表面,而密度泛函理论计算验证了表面结合的羟基自由基介导了吸附甘油中间碳的脱氢势垒;Ag纳米粒子促进了甘油中间羟基的选择性吸附,进一步诱导了甘油的选择性氧化。































 京公网安备 11010802027423号
京公网安备 11010802027423号