Journal of Hepatology ( IF 26.8 ) Pub Date : 2022-05-27 , DOI: 10.1016/j.jhep.2022.05.018 Laura Molina 1 , Junjie Zhu 2 , Eric Trépo 3 , Quentin Bayard 4 , Giuliana Amaddeo 5 , , Jean-Frédéric Blanc 6 , Julien Calderaro 7 , Xiaochao Ma 2 , Jessica Zucman-Rossi 8 , Eric Letouzé 9
|
Background & Aims
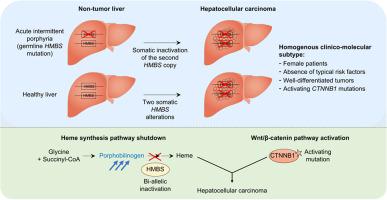
Acute intermittent porphyria (AIP), caused by heterozygous germline mutations of the heme synthesis pathway enzyme HMBS (hydroxymethylbilane synthase), confers a high risk of hepatocellular carcinoma (HCC) development. Yet, the role of HMBS in liver tumorigenesis remains unclear.
Methods
Herein, we explore HMBS alterations in a large series of 758 HCC cases, including 4 patients with AIP. We quantify the impact of HMBS mutations on heme biosynthesis pathway intermediates and we investigate the molecular and clinical features of HMBS-mutated tumors.
Results
We identify recurrent bi-allelic HMBS inactivation, both in patients with AIP acquiring a second somatic HMBS mutation and in sporadic HCC with 2 somatic hits. HMBS alterations are enriched in truncating mutations, in particular in splice regions, leading to abnormal transcript structures. Bi-allelic HMBS inactivation results in a massive accumulation of its toxic substrate porphobilinogen and synergizes with CTNNB1-activating mutations, leading to the development of well-differentiated tumors with a transcriptomic signature of Wnt/β-catenin pathway activation and a DNA methylation signature related to ageing. HMBS-inactivated HCC mostly affects females, in the absence of fibrosis and classical HCC risk factors.
Conclusions
These data identify HMBS as a tumor suppressor gene whose bi-allelic inactivation defines a homogenous clinical and molecular HCC subtype.
Lay summary
Heme (the precursor to hemoglobin, which plays a key role in oxygen transport around the body) synthesis occurs in the liver and involves several enzymes including hydroxymethylbilane synthase (HMBS). HMBS mutations cause acute intermittent porphyria, a disease caused by the accumulation of toxic porphyrin precursors. Herein, we show that HMBS inactivation is also involved in the development of liver cancers with distinct clinical and molecular characteristics.
中文翻译:

双等位基因羟甲基胆烷合酶失活定义了肝细胞癌的同质临床分子亚型
背景与目标
由血红素合成途径酶 HMBS(羟甲基胆烷合成酶)的杂合种系突变引起的急性间歇性卟啉症 (AIP)具有发生肝细胞癌 (HCC) 的高风险。然而,HMBS 在肝肿瘤发生中的作用仍不清楚。
方法
在此,我们探讨了 758 例 HCC 病例(包括 4 例 AIP 患者)中的 HMBS 改变。我们量化了HMBS突变对血红素生物合成途径中间体的影响,并研究了HMBS突变肿瘤的分子和临床特征。
结果
我们确定了复发性双等位基因 HMBS 失活,无论是在 AIP 患者中获得第二个体细胞HMBS突变还是在散发性 HCC 中具有 2 个体细胞命中。HMBS改变富含截断突变,特别是在剪接区,导致转录本结构异常。双等位基因 HMBS 失活导致其毒性底物胆色素原大量积累并与CTNNB1激活突变协同作用,导致分化良好的肿瘤的发展,其具有Wnt/β-连环蛋白通路激活的转录组学特征和 DNA 甲基化特征相关到老化。在没有纤维化和经典 HCC 危险因素的情况下,HMBS 灭活的 HCC 主要影响女性。
结论
这些数据将HMBS鉴定为肿瘤抑制基因,其双等位基因失活定义了同质临床和分子 HCC 亚型。
外行总结
血红素(血红蛋白的前体,在体内氧气运输中起着关键作用)在肝脏中合成,涉及多种酶,包括羟甲基胆碱合酶 (HMBS)。HMBS突变导致急性间歇性卟啉症,这是一种由有毒卟啉前体积累引起的疾病。在此,我们表明 HMBS 失活也参与具有不同临床和分子特征的肝癌的发展。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号