当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Precise O-Glycosylation Site Localization of CD24Fc by LC-MS Workflows
Analytical Chemistry ( IF 6.7 ) Pub Date : 2022-05-27 , DOI: 10.1021/acs.analchem.2c01137 Xiaojuan Li 1 , Robert Wilmanowski 2 , Xinliu Gao 1 , Zachary L VanAernum 1 , Daniel P Donnelly 1 , Brent Kochert 1 , Hillary A Schuessler 1 , Douglas Richardson 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2022-05-27 , DOI: 10.1021/acs.analchem.2c01137 Xiaojuan Li 1 , Robert Wilmanowski 2 , Xinliu Gao 1 , Zachary L VanAernum 1 , Daniel P Donnelly 1 , Brent Kochert 1 , Hillary A Schuessler 1 , Douglas Richardson 1
Affiliation
|
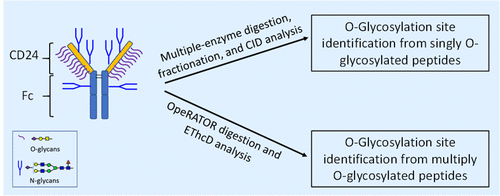
CD24Fc is a homodimeric recombinant Fc-fusion protein comprised of human CD24 connected to immunoglobulin G1 (IgG1) Fc fragment. CD24 is heavily glycosylated, and its biological function is considered mainly mediated by its glycosylation. Identification of the O-glycosylation sites would facilitate an in-depth understanding of the functional role of O-glycans in CD24. However, the presence of clustered mucin-type O-glycans together with N-glycans makes the determination of O-glycosylation sites and abundance very challenging. In this study, two sets of liquid chromatography-mass spectrometry (LC-MS) workflows were developed for the comprehensive characterization of O-glycosylation in CD24: (1) Fractionation and collision-induced dissociation (CID) workflow involving multienzyme digestion, fractionation, OpeRATOR/SialEXO digestion, and CID analysis; (2) Direct OpeRATOR/SialEXO digestion followed by electron-transfer/higher-energy collision dissociation (EThcD) analysis. The precise O-glycosylation sites were identified in CD24 for the first time, and the site occupancy was assessed. A total of 12 O-glycosylation sites were identified. Seven glycosylation sites were identified by both workflows, and five additional sites were identified only by the EThcD workflow. The predominant O-glycosylation site in CD24 was Thr25 followed by Thr15. The CID workflow provided an overall relative quantitation of O-glycoforms at the CD24 level and site localization for singly O-glycosylated peptides. The EThcD workflow directly identified glycosylation sites by tandem mass spectrometry (MS/MS) for singly, doubly, and triply O-glycosylated peptides. Together, both workflows validated each other’s results and can be applied to a complex mucin-type O-glycosylation site analysis of other glycoproteins and Fc-fusion therapeutics.
中文翻译:

通过 LC-MS 工作流程精确定位 CD24Fc 的 O-糖基化位点
CD24Fc 是一种同源二聚体重组 Fc 融合蛋白,由与免疫球蛋白 G1 (IgG1) Fc 片段连接的人 CD24 组成。CD24 是高度糖基化的,其生物学功能被认为主要由其糖基化介导。O-糖基化位点的鉴定将有助于深入了解 O-聚糖在 CD24 中的功能作用。然而,聚集粘蛋白型 O-聚糖和 N-聚糖的存在使得 O-糖基化位点和丰度的测定非常具有挑战性。在本研究中,开发了两组液相色谱-质谱 (LC-MS) 工作流程,用于全面表征 CD24 中的 O-糖基化:(1) 分馏和碰撞诱导解离 (CID) 工作流程,包括多酶消化、分馏、 Operator/SialEXO 消化和 CID 分析;(2) 直接 OpeRATOR/SialEXO 消化,然后进行电子转移/高能碰撞解离 (ETHcD) 分析。首次在CD24中确定了精确的O-糖基化位点,并评估了位点占用率。共鉴定了 12 个 O-糖基化位点。两个工作流程都鉴定了 7 个糖基化位点,另外 5 个位点仅通过 ETHcD 工作流程鉴定。CD24 中主要的 O-糖基化位点是 Thr25,其次是 Thr15。CID 工作流程提供了 CD24 水平上 O-糖型的总体相对定量和单 O-糖基化肽的位点定位。ETHcD 工作流程通过串联质谱 (MS/MS) 直接识别单、双和三 O-糖基化肽的糖基化位点。一起,
更新日期:2022-05-27
中文翻译:

通过 LC-MS 工作流程精确定位 CD24Fc 的 O-糖基化位点
CD24Fc 是一种同源二聚体重组 Fc 融合蛋白,由与免疫球蛋白 G1 (IgG1) Fc 片段连接的人 CD24 组成。CD24 是高度糖基化的,其生物学功能被认为主要由其糖基化介导。O-糖基化位点的鉴定将有助于深入了解 O-聚糖在 CD24 中的功能作用。然而,聚集粘蛋白型 O-聚糖和 N-聚糖的存在使得 O-糖基化位点和丰度的测定非常具有挑战性。在本研究中,开发了两组液相色谱-质谱 (LC-MS) 工作流程,用于全面表征 CD24 中的 O-糖基化:(1) 分馏和碰撞诱导解离 (CID) 工作流程,包括多酶消化、分馏、 Operator/SialEXO 消化和 CID 分析;(2) 直接 OpeRATOR/SialEXO 消化,然后进行电子转移/高能碰撞解离 (ETHcD) 分析。首次在CD24中确定了精确的O-糖基化位点,并评估了位点占用率。共鉴定了 12 个 O-糖基化位点。两个工作流程都鉴定了 7 个糖基化位点,另外 5 个位点仅通过 ETHcD 工作流程鉴定。CD24 中主要的 O-糖基化位点是 Thr25,其次是 Thr15。CID 工作流程提供了 CD24 水平上 O-糖型的总体相对定量和单 O-糖基化肽的位点定位。ETHcD 工作流程通过串联质谱 (MS/MS) 直接识别单、双和三 O-糖基化肽的糖基化位点。一起,

































 京公网安备 11010802027423号
京公网安备 11010802027423号