International Journal for Parasitology: Drugs and Drug Resistance ( IF 4.1 ) Pub Date : 2022-05-26 , DOI: 10.1016/j.ijpddr.2022.05.004 Yang Zheng 1 , Joachim Müller 2 , Stefan Kunz 3 , Marco Siderius 1 , Louis Maes 4 , Guy Caljon 4 , Norbert Müller 2 , Andrew Hemphill 2 , Geert Jan Sterk 1 , Rob Leurs 1
|
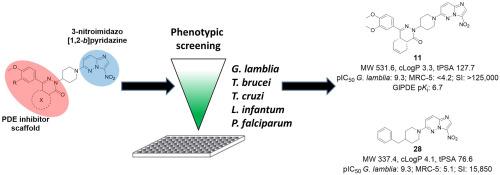
As there is a continuous need for novel anti-infectives, the present study aimed to fuse two modes of action into a novel 3-nitroimidazo[1,2-b]pyridazine scaffold to improve antiparasitic efficacy. For this purpose, we combined known structural elements of phosphodiesterase inhibitors, a target recently proposed for Trypanosoma brucei and Giardia lamblia, with a nitroimidazole scaffold to generate nitrosative stress. The compounds were evaluated in vitro against a panel of protozoal parasites, namely Giardia lamblia, Trypanosoma brucei, T. cruzi, Leishmania infantum and Plasmodium falciparum and for cytotoxicity on MRC-5 cells. Interestingly, selective sub-nanomolar activity was obtained against G. lamblia, and by testing several analogues with and without the nitro group, it was shown that the presence of a nitro group, but not PDE inhibition, is responsible for the low IC50 values of these novel compounds. Adding the favourable drug-like properties (low molecular weight, cLogP (1.2–4.1) and low polar surface area), the key compounds from the 3-nitroimidazo[1,2-b]pyridazine series can be considered as valuable hits for further anti-giardiasis drug exploration and development.
中文翻译:

3-硝基咪唑并[1,2-b]哒嗪作为具有亚纳摩尔抗贾第鞭毛虫活性的新型抗寄生虫支架
由于对新型抗感染药物的持续需求,本研究旨在将两种作用模式融合到新型 3-硝基咪唑并[1,2- b ]哒嗪支架中,以提高抗寄生虫功效。为此,我们将磷酸二酯酶抑制剂的已知结构元素(最近提出的针对布氏锥虫和贾第鞭毛虫的靶标)与硝基咪唑支架结合以产生亚硝化应激。这些化合物在体外针对一组原生动物寄生虫进行了评估,即兰氏贾第鞭毛虫、布氏锥虫、克氏锥虫、婴儿利什曼原虫和恶性疟原虫以及对 MRC-5 细胞的细胞毒性。有趣的是,获得了针对G. lamblia的选择性亚纳摩尔活性,并且通过测试具有和不具有硝基的几种类似物,表明硝基的存在而非 PDE 抑制是低 IC 50值的原因这些新颖的化合物。3-硝基咪唑并[1,2- b ]哒嗪系列的关键化合物具有良好的类药物特性(低分子量、cLogP (1.2–4.1) 和低极性表面积),可被视为进一步研究的有价值的产物。抗贾第虫病药物的探索与开发。






























 京公网安备 11010802027423号
京公网安备 11010802027423号