当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility and Solvent Effect Analysis of Boc-l-glutamine in Different Solvents at Multiple Temperatures
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-05-26 , DOI: 10.1021/acs.jced.2c00162 Dandan Liu , Jingxuan Qiu , Shen Hu , Yue Zhao , Peng Wang , Zhenyu Li
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-05-26 , DOI: 10.1021/acs.jced.2c00162 Dandan Liu , Jingxuan Qiu , Shen Hu , Yue Zhao , Peng Wang , Zhenyu Li
|
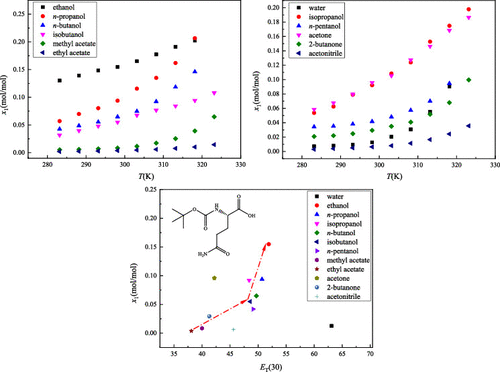
The solubility behavior of Boc-l-glutamine in water, ethanol, n-propanol, isopropanol, n-butanol, isobutanol, n-pentanol, acetone, 2-butanone, acetonitrile, methyl acetate, and ethyl acetate was investigated by the static gravimetric method at 98.8 kPa and multiple temperatures from 283.15 to 323.15 K. The equilibrium solid-phase characterization of Boc-l-glutamine in different solvent systems was carried out by the powder X-ray diffraction analysis. In 12 pure solvents, the solubility increased with absolute temperature, and the maximum value was recorded in ethanol and the minimum in ethyl acetate. The solubility behavior and solvent effects in different solvents were first analyzed by the empirical solvent polarity parameters (ET(30)) as the main factor and then interpreted by hydrogen bond acceptor tendencies, cohesive energy density, structural similarity, steric effect, and solvent viscosity for some exceptions respectively. Furthermore, the Yaws model and the modified Apelblat model were employed to fit the solubility data. Akaike Information Criterion and Akaike weights were calculated to evaluate the two solubility models. The results show that the modified Apelblat model has better correlation results than the Yaws model. The results could provide a theoretical basis for the production and application of Boc-l-glutamine in organic or biological process development.
中文翻译:

Boc-l-谷氨酰胺在不同溶剂中不同温度下的溶解度及溶剂效应分析
采用静态重量法研究了Boc -l-谷氨酰胺在水、乙醇、正丙醇、异丙醇、正丁醇、异丁醇、正戊醇、丙酮、2-丁酮、乙腈、乙酸甲酯和乙酸乙酯中的溶解行为方法在 98.8 kPa 和从 283.15 到 323.15 K 的多个温度。 Boc- l的平衡固相表征采用粉末X射线衍射分析法对不同溶剂体系中的-谷氨酰胺进行了分析。在12种纯溶剂中,溶解度随绝对温度的升高而增加,在乙醇中最大,在乙酸乙酯中最小。首先通过经验溶剂极性参数(E T(30)) 作为主要因素,然后分别通过氢键受体倾向、内聚能密度、结构相似性、空间效应和溶剂粘度来解释某些例外情况。此外,采用 Yaws 模型和改进的 Apelblat 模型来拟合溶解度数据。计算 Akaike 信息标准和 Akaike 权重以评估两种溶解度模型。结果表明,修正后的Apelblat模型比Yaws模型具有更好的相关性结果。研究结果可为Boc -l-谷氨酰胺的生产和在有机或生物工艺开发中的应用提供理论依据。
更新日期:2022-05-26
中文翻译:

Boc-l-谷氨酰胺在不同溶剂中不同温度下的溶解度及溶剂效应分析
采用静态重量法研究了Boc -l-谷氨酰胺在水、乙醇、正丙醇、异丙醇、正丁醇、异丁醇、正戊醇、丙酮、2-丁酮、乙腈、乙酸甲酯和乙酸乙酯中的溶解行为方法在 98.8 kPa 和从 283.15 到 323.15 K 的多个温度。 Boc- l的平衡固相表征采用粉末X射线衍射分析法对不同溶剂体系中的-谷氨酰胺进行了分析。在12种纯溶剂中,溶解度随绝对温度的升高而增加,在乙醇中最大,在乙酸乙酯中最小。首先通过经验溶剂极性参数(E T(30)) 作为主要因素,然后分别通过氢键受体倾向、内聚能密度、结构相似性、空间效应和溶剂粘度来解释某些例外情况。此外,采用 Yaws 模型和改进的 Apelblat 模型来拟合溶解度数据。计算 Akaike 信息标准和 Akaike 权重以评估两种溶解度模型。结果表明,修正后的Apelblat模型比Yaws模型具有更好的相关性结果。研究结果可为Boc -l-谷氨酰胺的生产和在有机或生物工艺开发中的应用提供理论依据。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号