Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2022-05-26 , DOI: 10.1016/j.bmcl.2022.128820 Jinsu Bae 1 , Koon Mook Kang 2 , Yong-Chul Kim 3
|
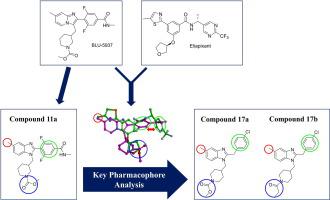
Drug discovery programs targeting P2X3 receptors (P2X3R), an extracellular adenosine 5′-triphosphate (ATP) gated cation channel family, have been actively investigated for several CNS-related diseases. The current unmet need in the field of P2X3R targeted drugs is to avoid a side effect, the loss of taste, that could be reduced by increase of the P2X3R selectivity vs P2X2/3R. In this study, 5-methyl-1H-benzo[d]imidazole derivatives were designed and synthesized from the analysis of key pharmacophores of current antagonists. In the structure–activity relationship study, the most potent compounds 17a-b was discovered as potent P2X3R antagonists with IC50 values of 145 and 206 nM, and selectivity index of 60 and 41, respectively. In addition, 17a-b showed the not-competitive antagonism, but poor binding score in the docking study at the known allosteric binding site of Gefapixant binding site, indicating that another allosteric binding site might be existing for the novel P2X3R antagonists.
中文翻译:

5-甲基-1H-苯并[d]咪唑衍生物作为新型 P2X3 受体拮抗剂的发现
针对 P2X3 受体 (P2X3R) 的药物发现计划是一种细胞外腺苷 5'-三磷酸 (ATP) 门控阳离子通道家族,已针对几种 CNS 相关疾病进行了积极研究。P2X3R 靶向药物领域当前未满足的需求是避免副作用,即味觉丧失,这可以通过增加 P2X3R 相对于 P2X2/3R 的选择性来减少。在本研究中,通过对当前拮抗剂的关键药效团的分析,设计并合成了5-methyl-1 H - benzo[ d ]imidazole 衍生物。在构效关系研究中,最有效的化合物17a - b被发现是具有 IC 50的有效 P2X3R 拮抗剂值分别为 145 和 206 nM,选择性指数分别为 60 和 41。此外,17a - b在Gefapixant结合位点的已知变构结合位点的对接研究中显示出非竞争性拮抗作用,但结合评分较差,表明新型P2X3R拮抗剂可能存在另一个变构结合位点。









































 京公网安备 11010802027423号
京公网安备 11010802027423号