当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
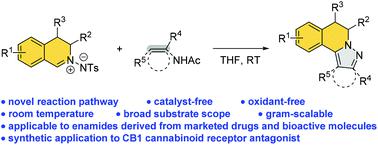
Catalyst-free and oxidant-free tandem aza-Mannich/cyclization/aromatization of C,N-cyclic azomethine imines with enamides: facile synthesis of 5,6-dihydropyrazolo[5,1-a]isoquinolines
Green Chemistry ( IF 9.3 ) Pub Date : 2022-05-25 , DOI: 10.1039/d2gc01275h Hao Dong 1 , Yongxing Zhang 1 , Xiaochen Tian 1 , Ruochen Pang 1 , Weiwu Ren 1, 2 , Yang Wang 1, 2
Green Chemistry ( IF 9.3 ) Pub Date : 2022-05-25 , DOI: 10.1039/d2gc01275h Hao Dong 1 , Yongxing Zhang 1 , Xiaochen Tian 1 , Ruochen Pang 1 , Weiwu Ren 1, 2 , Yang Wang 1, 2
Affiliation
|
A novel and efficient catalyst-free and oxidant-free tandem aza-Mannich/cyclization/aromatization reaction of C,N-cyclic azomethine imines with enamides has been developed. This practical one-step protocol enables a simple and environmentally friendly route toward the straightforward synthesis of highly substituted 5,6-dihydropyrazolo[5,1-a]isoquinolines. Different types of enamides and enamines, especially enamides derived from marketed drugs as well as bioactive molecules, are suitable substrates. CB1 cannabinoid receptor antagonist could be synthesized efficiently based on this methodology, illustrating that this would be a practical strategy to synthesize valuable structural motifs.
中文翻译:

无催化剂和无氧化剂串联氮杂曼尼希/环化/芳构化 C,N-环偶氮甲亚胺与烯酰胺:轻松合成 5,6-二氢吡唑并[5,1-a]异喹啉
开发了一种新型高效的无催化剂、无氧化剂串联氮杂曼尼希/环化/芳构化C , N-环偶氮甲亚胺与烯酰胺反应。这种实用的一步法为直接合成高度取代的 5,6-二氢吡唑并[5,1- a ] 异喹啉提供了一种简单且环保的途径。不同类型的烯酰胺和烯胺,尤其是衍生自上市药物以及生物活性分子的烯酰胺,是合适的底物。CB1 大麻素受体拮抗剂可以基于该方法有效合成,说明这将是合成有价值的结构基序的实用策略。
更新日期:2022-05-25
中文翻译:

无催化剂和无氧化剂串联氮杂曼尼希/环化/芳构化 C,N-环偶氮甲亚胺与烯酰胺:轻松合成 5,6-二氢吡唑并[5,1-a]异喹啉
开发了一种新型高效的无催化剂、无氧化剂串联氮杂曼尼希/环化/芳构化C , N-环偶氮甲亚胺与烯酰胺反应。这种实用的一步法为直接合成高度取代的 5,6-二氢吡唑并[5,1- a ] 异喹啉提供了一种简单且环保的途径。不同类型的烯酰胺和烯胺,尤其是衍生自上市药物以及生物活性分子的烯酰胺,是合适的底物。CB1 大麻素受体拮抗剂可以基于该方法有效合成,说明这将是合成有价值的结构基序的实用策略。








































 京公网安备 11010802027423号
京公网安备 11010802027423号