当前位置:
X-MOL 学术
›
Appl. Organomet. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Substituent effects of tertiary phosphines on the structures and electrochemical performances of azadithiolato-bridged diiron model complexes of [FeFe]-hydrogenases
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2022-05-24 , DOI: 10.1002/aoc.6751 Xu‐Feng Liu 1 , Zhong‐Yi Ma 2 , Bo Jin 2 , Dong Wang 2 , Pei‐Hua Zhao 2
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2022-05-24 , DOI: 10.1002/aoc.6751 Xu‐Feng Liu 1 , Zhong‐Yi Ma 2 , Bo Jin 2 , Dong Wang 2 , Pei‐Hua Zhao 2
Affiliation
|
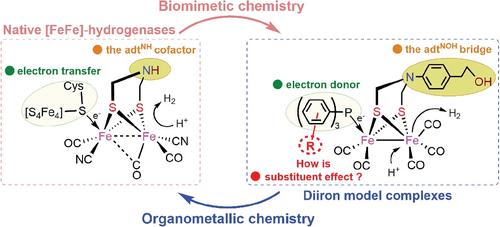
In this work, a new series of azadithiolato-bridged diiron complexes [Fe2(μ-adtNOH)(CO)5{P(C6H4R)3}] (adtNOH = (SCH2)2N(C6H4CH2CH2OH-p) and R = Cl-p, 1; Me-m, 2; OMe-p, 3 supported by tertiary phosphines, which may be considered as the active site models of [FeFe]-hydrogenases, has been prepared in 73–81% yields by the Me3NO-assisted decarbonylating reactions of all-CO precursor [Fe2(μ-adtNOH)(CO)6] (A) with several tertiary phosphines [(P(C6H4R)3] in MeCN at room temperature. All the new complexes 1–3 are fully characterized by elemental analysis, spectroscopic techniques (Fourier transform-infrared spectroscopy [FT-IR] and nuclear magnetic resonance [NMR]), and especially for 1 and 3 by X-ray crystallography. The IR and 31P{1H} NMR spectroscopic analyses have shown that the electron density of diiron center in 1–3 may be adjusted by the introduction of the different substituents (R) of the P(C6H4R)3 ligands into the [2Fe2S] cluster. The X-ray crystallographic investigation has displayed that the typical Fe-Fe, Fe-P, and Fe-C distances of 1–3 are significantly influenced by the electronic effects of the P(C6H4R)3 phosphines coordinated apically to one Fe atom, whereas their Papical-Fe-Fe and Capical-Fe-Fe angles are mainly controlled by the steric interaction between the phosphine ligands and the dithiolate bridges. Further, the electrochemical performances of 1–3 are studied and compared in the absence or presence of acetic acid (HOAc) as proton source by using cyclic voltammetry, suggesting that they are active for the electrocatalytic proton reduction to hydrogen (H2).
中文翻译:

叔膦取代基对[FeFe]-氢化酶氮杂二硫醇桥联二铁模型配合物结构和电化学性能的影响
在这项工作中,一系列新的氮杂二硫醇桥联二铁配合物 [Fe 2 ( μ -adt NOH )(CO) 5 {P(C 6 H 4 R) 3 }] (adt NOH = (SCH 2 ) 2 N(C 6 H 4 CH 2 CH 2 OH- p ) 和 R = Cl- p , 1 ; Me- m , 2 ; OMe- p , 3由叔膦支持,可以被认为是[FeFe]-氢化酶的活性位点模型,通过Me 3 NO 辅助的全CO前体[Fe 2 ( μ - adt NOH )(CO) 6 ] ( A ) 与几种叔膦 [(P(C 6 H 4 R) 3 ] 在 MeCN 中在室温下。所有新的配合物1 – 3都通过元素分析、光谱技术 (傅里叶变换红外光谱 [FT-IR] 和核磁共振 [NMR]),特别是对于1和3 X 射线晶体学。IR和31 P{ 1 H} NMR光谱分析表明,可以通过引入P(C 6 H 4 R) 3配体的不同取代基(R)来调节1-3中二铁中心的电子密度进入 [2Fe2S] 簇。X 射线晶体学研究表明,典型的 Fe-Fe、Fe-P 和 Fe-C 距离1 – 3受到顶部配位的 P(C 6 H 4 R) 3膦的电子效应的显着影响一个 Fe 原子,而它们的 P顶端-Fe-Fe 和 C顶端-Fe-Fe 角主要由膦配体和二硫醇盐桥之间的空间相互作用控制。此外,通过使用循环伏安法研究和比较了在不存在或存在乙酸(HOAc)作为质子源的情况下1-3的电化学性能,表明它们对电催化质子还原为氢(H 2)具有活性。
更新日期:2022-05-24
中文翻译:

叔膦取代基对[FeFe]-氢化酶氮杂二硫醇桥联二铁模型配合物结构和电化学性能的影响
在这项工作中,一系列新的氮杂二硫醇桥联二铁配合物 [Fe 2 ( μ -adt NOH )(CO) 5 {P(C 6 H 4 R) 3 }] (adt NOH = (SCH 2 ) 2 N(C 6 H 4 CH 2 CH 2 OH- p ) 和 R = Cl- p , 1 ; Me- m , 2 ; OMe- p , 3由叔膦支持,可以被认为是[FeFe]-氢化酶的活性位点模型,通过Me 3 NO 辅助的全CO前体[Fe 2 ( μ - adt NOH )(CO) 6 ] ( A ) 与几种叔膦 [(P(C 6 H 4 R) 3 ] 在 MeCN 中在室温下。所有新的配合物1 – 3都通过元素分析、光谱技术 (傅里叶变换红外光谱 [FT-IR] 和核磁共振 [NMR]),特别是对于1和3 X 射线晶体学。IR和31 P{ 1 H} NMR光谱分析表明,可以通过引入P(C 6 H 4 R) 3配体的不同取代基(R)来调节1-3中二铁中心的电子密度进入 [2Fe2S] 簇。X 射线晶体学研究表明,典型的 Fe-Fe、Fe-P 和 Fe-C 距离1 – 3受到顶部配位的 P(C 6 H 4 R) 3膦的电子效应的显着影响一个 Fe 原子,而它们的 P顶端-Fe-Fe 和 C顶端-Fe-Fe 角主要由膦配体和二硫醇盐桥之间的空间相互作用控制。此外,通过使用循环伏安法研究和比较了在不存在或存在乙酸(HOAc)作为质子源的情况下1-3的电化学性能,表明它们对电催化质子还原为氢(H 2)具有活性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号