Nano Research ( IF 9.5 ) Pub Date : 2022-05-24 , DOI: 10.1007/s12274-022-4359-6 Daoming Zhu , Ruoyu Ling , Hao Chen , Meng Lyu , Haisheng Qian , Konglin Wu , Guoxin Li , Xianwen Wang
|
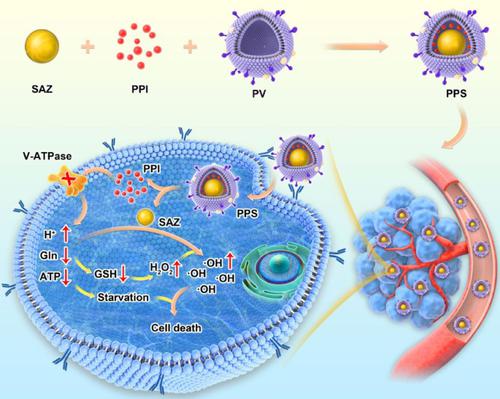
Single-atom nanozymes (SAZs) with peroxidase (POD)-like activity have good nanocatalytic tumor therapy (NCT) capabilities. However, insufficient hydrogen peroxide (H2O2) and hydrogen ions in the cells limit their therapeutic effects. Herein, to overcome these limitations, a biomimetic single-atom nanozyme system was developed for self-enhanced NCT. We used a previously described approach to produce platelet membrane vesicles. Using a high-temperature carbonization approach, copper SAZs with excellent POD-like activity were successfully synthesized. Finally, through physical extrusion, a proton pump inhibitor (PPI; pantoprazole sodium) and the SAZs were combined with platelet membrane vesicles to create PPS. Both in vivo and in vitro, PPS displayed good tumor-targeting and accumulation abilities. PPIs were able to simultaneously regulate the hydrogen ion, glutathione (GSH), and H2O2 content in tumor cells, significantly improve the catalytic ability of SAZs, and achieve self-enhanced NCT. Our in vivo studies showed that PPS had a tumor suppression rate of > 90%. PPS also limited the synthesis of GSH in cells at the source; thus, glutamine metabolism therapy and NCT were integrated into an innovative method, which provides a novel strategy for multimodal tumor therapy.
中文翻译:

用于自增强纳米催化肿瘤治疗的仿生铜单原子纳米酶系统
具有类似过氧化物酶 (POD) 活性的单原子纳米酶 (SAZ) 具有良好的纳米催化肿瘤治疗 (NCT) 能力。然而,细胞中的过氧化氢(H 2 O 2)和氢离子不足限制了它们的治疗效果。在此,为了克服这些限制,开发了一种用于自增强 NCT 的仿生单原子纳米酶系统。我们使用先前描述的方法来生产血小板膜囊泡。采用高温碳化方法,成功合成了具有优异类 POD 活性的铜 SAZ。最后,通过物理挤压,质子泵抑制剂(PPI;泮托拉唑钠)和 SAZ 与血小板膜囊泡结合形成 PPS。体内和体外_, PPS displayed good tumor-targeting and accumulation abilities. PPIs were able to simultaneously regulate the hydrogen ion, glutathione (GSH), and H2O2 content in tumor cells, significantly improve the catalytic ability of SAZs, and achieve self-enhanced NCT. Our in vivo studies showed that PPS had a tumor suppression rate of > 90%. PPS also limited the synthesis of GSH in cells at the source; thus, glutamine metabolism therapy and NCT were integrated into an innovative method, which provides a novel strategy for multimodal tumor therapy.
















































 京公网安备 11010802027423号
京公网安备 11010802027423号