当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transition-Metal-Free Alkylative Aromatization of Tetralone Using Alcohol/Amino Alcohol towards the Synthesis of Bioactive Naphthol and Benzo[e/g]indole Derivatives
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-05-25 , DOI: 10.1021/acs.joc.2c00779 Akash S Ubale 1 , Gokul S Londhe 1 , Moseen A Shaikh 1 , Boopathy Gnanaprakasam 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-05-25 , DOI: 10.1021/acs.joc.2c00779 Akash S Ubale 1 , Gokul S Londhe 1 , Moseen A Shaikh 1 , Boopathy Gnanaprakasam 1
Affiliation
|
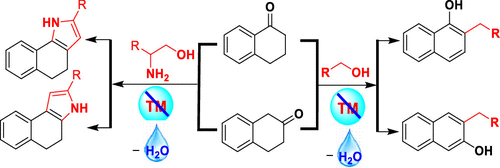
Herein, we report alkylative aromatization of tetralone for the synthesis of bioactive naphthols and benzo[e/g]indole derivatives using alcohols in the presence of NaOH via an aerobic oxidative cross-coupling protocol. This is a general and transition-metal-free method, which uses an inexpensive base, avoids inert conditions, and furnishes water and hydrogen peroxide as the byproducts. Moreover, this method demonstrated with wide substrate scope and obtained exclusive regioselectivity.
中文翻译:

使用醇/氨基醇对四氢萘酮进行无过渡金属烷基芳构化合成生物活性萘酚和苯并[e/g]吲哚衍生物
在此,我们报告了四氢萘酮的烷基芳构化,用于在NaOH 存在下通过需氧氧化交叉偶联方案使用醇合成生物活性萘酚和苯并[ e / g ]吲哚衍生物。这是一种通用且不含过渡金属的方法,它使用廉价的碱,避免惰性条件,并提供水和过氧化氢作为副产品。此外,该方法具有广泛的底物范围,并获得了独有的区域选择性。
更新日期:2022-05-25
中文翻译:

使用醇/氨基醇对四氢萘酮进行无过渡金属烷基芳构化合成生物活性萘酚和苯并[e/g]吲哚衍生物
在此,我们报告了四氢萘酮的烷基芳构化,用于在NaOH 存在下通过需氧氧化交叉偶联方案使用醇合成生物活性萘酚和苯并[ e / g ]吲哚衍生物。这是一种通用且不含过渡金属的方法,它使用廉价的碱,避免惰性条件,并提供水和过氧化氢作为副产品。此外,该方法具有广泛的底物范围,并获得了独有的区域选择性。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号