Synthesis ( IF 2.2 ) Pub Date : 2022-05-23 , DOI: 10.1055/s-0040-1719925
Chong-Dao Lu 1, 2 , Nuermaimaiti Yisimayili 1, 3 , Li-Feng Chu 2 , Jie Feng 1, 3
|
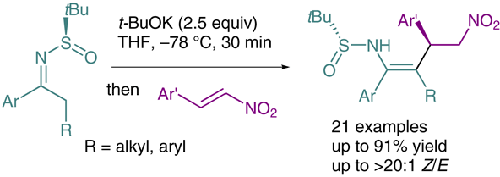
N-Sulfinyl metalloenamines, generated by deprotonating α-linear N-tert-butanesulfinyl ketimines, reacted with nitroalkenes via stereoselective conjugate addition to give Michael adducts with opposite stereochemistry to that obtained using α-branched sulfinylketimines. In the presence of excess base (2.5 equiv t-BuOK), the adducts derived from α-linear ketimines were further stereoselectively deprotonated to afford the corresponding kinetically favorable N-sulfinyl (Z)-enamine derivatives in good yields with good stereoselectivities. A reaction model was proposed to rationalize the observed stereochemistry.
中文翻译:

α-线性 N-叔丁亚磺酰基酮亚胺与硝基烯烃的立体选择性共轭加成-烯化
N-亚磺酰基金属烯胺是通过对 α-线性N-叔丁亚磺酰基酮亚胺进行去质子化生成的,通过立体选择性共轭加成与硝基烯烃反应,得到具有与使用 α-支链亚磺酰基酮亚胺相反的立体化学的迈克尔加合物。在过量碱(2.5 equiv t -BuOK)存在下,衍生自 α-线性酮亚胺的加合物进一步立体选择性去质子化,以良好的收率和良好的立体选择性提供相应的动力学有利的N-亚磺酰基 ( Z )-烯胺衍生物。提出了一种反应模型来合理化观察到的立体化学。

































 京公网安备 11010802027423号
京公网安备 11010802027423号