Chemosphere ( IF 8.1 ) Pub Date : 2022-05-20 , DOI: 10.1016/j.chemosphere.2022.135020 Xiangyu Li 1 , Gang Yu 1 , Yujue Wang 1
|
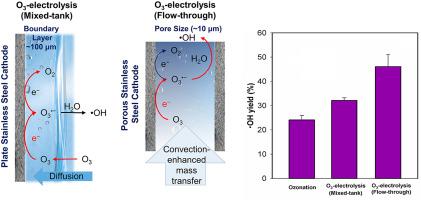
In this study, a flow-through ozone-electrolysis (O3-electrolysis) process was developed by combining ozonation with an electrolysis using a porous reactive electrochemical membrane (REM) cathode. Due to the convection-enhanced mass transport and fast radial diffusion inside the small pores of REM cathodes, the rate of cathodic O3 reduction to ozonide radicals (O3•−) was significantly enhanced, while the further cathodic O3•− reduction to oxygen was inhibited during the flow-through O3-electrolysis process compared to the conventional mixed-tank O3-electrolysis process. Consequently, more hydroxyl radicals (•OH) were formed from O3•− decay in water during the flow-through O3-electrolysis process than the mixed-tank O3-electrolysis process. Corresponding to the higher •OH yields from cathodic O3 reduction, the flow-through O3-electrolysis process substantially enhanced the abatement kinetics and efficiency of para-benzoic acid (pCBA, a model compound of ozone-resistant micropollutant) in a groundwater than conventional ozonation and the mixed-tank O3-electrolysis process. These results suggest that the flow-through O3-electrolysis process may provide a competitive treatment technology for micropollutant abatement in water treatment.
中文翻译:

流通式反应电化学膜阴极在臭氧电解过程中增强阴极臭氧还原产生的羟基自由基
在这项研究中,通过将臭氧化与使用多孔反应电化学膜 (REM) 阴极的电解相结合,开发了流通式臭氧电解 (O 3 - 电解) 工艺。由于 REM 正极小孔内的对流增强传质和快速径向扩散,阴极 O 3还原为臭氧自由基(O 3 •-)的速率显着提高,而进一步的阴极 O 3 •-还原为与传统的混合槽O 3 -电解过程相比,在流通式O 3 -电解过程中氧气受到抑制。因此,更多的羟基自由基(•OH)由O 3形成•−流通式O 3 -电解过程中的水中衰减比混合槽O 3 -电解过程中的衰减。与阴极 O 3还原产生的较高 •OH 产率相对应,流通式 O 3电解过程显着提高了地下水中对苯甲酸(p CBA,一种抗臭氧微污染物的模型化合物)的减排动力学和效率优于传统的臭氧化和混合罐O 3电解工艺。这些结果表明,流通式O 3电解工艺可以为水处理中的微污染物减排提供有竞争力的处理技术。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号