Acta Pharmaceutica Sinica B ( IF 14.7 ) Pub Date : 2022-05-20 , DOI: 10.1016/j.apsb.2022.05.017 Ping Bai 1 , Prasenjit Mondal 2 , Frederick A Bagdasarian 1 , Nisha Rani 1 , Yan Liu 1 , Ashley Gomm 2 , Darcy R Tocci 1 , Se Hoon Choi 2 , Hsiao-Ying Wey 1 , Rudolph E Tanzi 2 , Can Zhang 2 , Changning Wang 1
|
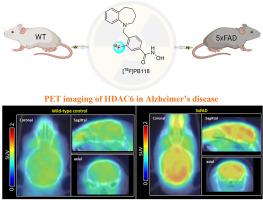
Although the epigenetic regulatory protein histone deacetylase 6 (HDAC6) has been recently implicated in the etiology of Alzheimer's disease (AD), little is known about the role of HDAC6 in the etiopathogenesis of AD and whether HDAC6 can be a potential therapeutic target for AD. Here, we performed positron emission tomography (PET) imaging in combination with histopathological analysis to better understand the underlying pathomechanisms of HDAC6 in AD. We first developed [18F]PB118 which was demonstrated as a valid HDAC6 radioligand with excellent brain penetration and high specificity to HDAC6. PET studies of [18F]PB118 in 5xFAD mice showed significantly increased radioactivity in the brain compared to WT animals, with more pronounced changes identified in the cortex and hippocampus. The translatability of this radiotracer for AD in a potential human use was supported by additional studies, including similar uptake profiles in non-human primates, an increase of HDAC6 in AD-related human postmortem hippocampal tissues by Western blotting protein analysis, and our ex vivo histopathological analysis of HDAC6 in postmortem brain tissues of our animals. Collectively, our findings show that HDAC6 may lead to AD by mechanisms that tend to affect brain regions particularly susceptible to AD through an association with amyloid pathology.
中文翻译:

开发用于阿尔茨海默病 HDAC6 成像的潜在 PET 探针
尽管表观遗传调节蛋白组蛋白脱乙酰酶 6 (HDAC6) 最近与阿尔茨海默病 (AD) 的病因有关,但人们对 HDAC6 在 AD 发病机制中的作用以及 HDAC6 是否可以成为 AD 的潜在治疗靶点知之甚少。在这里,我们结合组织病理学分析进行了正电子发射断层扫描 (PET) 成像,以更好地了解 AD 中 HDAC6 的潜在病理机制。我们首先开发了 [ 18 F]PB118,它被证明是一种有效的 HDAC6 放射性配体,具有出色的脑渗透性和对 HDAC6 的高特异性。[ 18的 PET 研究与 WT 动物相比,5xFAD 小鼠中的 F]PB118 在大脑中显示出显着增加的放射性,在皮质和海马体中发现了更明显的变化。这种用于 AD 的放射性示踪剂在人类潜在用途中的可转化性得到了其他研究的支持,包括在非人类灵长类动物中的类似摄取曲线,通过蛋白质印迹蛋白分析在 AD 相关的人类死后海马组织中增加 HDAC6,以及我们的离体我们动物死后脑组织中 HDAC6 的组织病理学分析。总的来说,我们的研究结果表明,HDAC6 可能通过与淀粉样蛋白病理学相关的机制导致 AD,这种机制往往会影响特别易受 AD 影响的大脑区域。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号