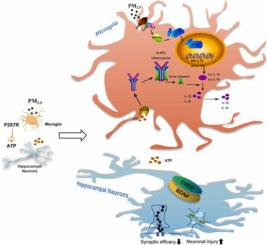
神经炎症是PM 2.5诱发认知障碍的关键机制,活化的小胶质细胞在此过程中起重要作用。然而,由 PM 2.5诱导的活化小胶质细胞损害海马神经元的机制尚未完全阐明。在这项研究中,我们专注于 HMGB1-NLRP3-P2X7R 通路在 PM 2.5诱导的海马神经元损伤中介导小胶质细胞活化的作用,使用小胶质细胞和海马神经元的共培养模型。我们发现 PM 2.5导致激活的小胶质细胞和 HMGB1-NLRP3 炎症通路,并以剂量依赖性方式升高 IL-18 和 IL-1β 的促炎细胞因子。值得注意的是,我们接下来利用先前报道的 HMGB1 药理学抑制剂或 siRNA,发现它们显着抑制了源自 PM 2.5的下游 NLRP3 和 MAPK 通路的激活暴露,并下调小胶质细胞中的 IL-18 和 IL-1β。此外,我们采用共培养的海马神经元和小胶质细胞,发现减少 HMGB1 显着降低神经元损伤、cl-caspase3 凋亡相关蛋白、突触损伤和 5-HT2A 神经递质受体,以及显着升高的突触前和突触后蛋白 SYP和 PSD-95,以及 p-CREB 和 BDNF 的学习和记忆相关蛋白。PM 2.5引起的神经元损伤在同时使用 HMGB1 siRNA 和 NLRP3 激动剂的情况下无法避免。在小胶质细胞中单独沉默 NLRP3 后,海马神经元表现出过度自噬减少和 GAP43 突触蛋白以及 NCAM1 学习和记忆相关蛋白上调。因此,我们进一步研究了 PM 2.5暴露下海马神经元如何影响小胶质细胞,进一步研究表明,沉默 HMGB1 可以影响 P2X7R 的激活并减少海马神经元 ATP 的释放,从而保护小胶质细胞和海马神经元之间的相互作用。目前的工作表明,调节 HMGB1-NLRP3-P2X7R 通路可以抑制 PM 2.5诱导的小胶质细胞活化。减轻海马神经元损伤,稳定小胶质细胞与神经元之间的微环境。这有助于维持海马神经元的正常功能并减轻因 PM 2.5暴露而导致的认知障碍。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
HMGB1-NLRP3-P2X7R pathway participates in PM2.5-induced hippocampal neuron impairment by regulating microglia activation
Neuroinflammation is a key mechanism underlying the cognitive impairment induced by PM2.5, and activated microglia plays an important role in this process. However, the mechanisms by which activated microglia induced by PM2.5 impair hippocampal neurons have not been fully elucidated. In this study, we focused on the role of HMGB1-NLRP3-P2X7R pathway which mediated the microglia activation in hippocampal neurons impairment induced by PM2.5 using a co-culture model of microglia and hippocampal neurons. We found that PM2.5 resulted in activated microglia and HMGB1-NLRP3 inflammatory pathway, and elevated proinflammatory cytokines of IL-18 and IL-1β in a dose-dependent manner. Notably, we next utilized previously reported pharmacological inhibitors or siRNA for HMGB1 and found that they significantly inhibited the activation of downstream NLRP3 and MAPK pathways derived from PM2.5 exposure, and down-regulated IL-18 and IL-1β in microglia. Furthermore, we employed co-cultured hippocampal neurons and microglia and found that reducing HMGB1 significantly decreased neuron impairment, apoptosis related protein of cl-caspase3, synaptic damage, and neurotransmitter receptor of 5-HT2A, along with notably elevated presynaptic and postsynaptic proteins of SYP and PSD-95, as well as learning and memory related proteins of p-CREB and BDNF. The neuronal impairment induced by PM2.5 could not be prevented in the case of simultaneous employment of HMGB1 siRNA and NLRP3 agonist. After silencing NLRP3 alone in microglia, hippocampal neurons demonstrated decreased excessive autophagy and up-regulated synaptic protein of GAP43 as well as learning and memory related protein of NCAM1. Therefore, we further studied how hippocampal neurons affected microglia under PM2.5 exposure, Further investigation indicated that silencing HMGB1 could affect the activation of P2X7R and reduce the release of ATP from hippocampal neurons, thus protecting the interaction between microglia and hippocampal neurons. The present work suggests that regulation of HMGB1-NLRP3-P2X7R pathway can inhibit the microglia activation induced by PM2.5 to alleviate hippocampal neuron impairment and stabilize the microenvironment between microglia and neurons. This contributes to maintaining the normal function of hippocampal neurons and alleviating the cognitive impairment derived from PM2.5 exposure.


































 京公网安备 11010802027423号
京公网安备 11010802027423号