Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2022-05-21 , DOI: 10.1016/j.jhazmat.2022.129189 Shaoyang Hu 1 , Xiao Chen 2 , Beibei Zhang 3 , Lanyao Liu 4 , Tingting Gong 5 , Qiming Xian 6
|
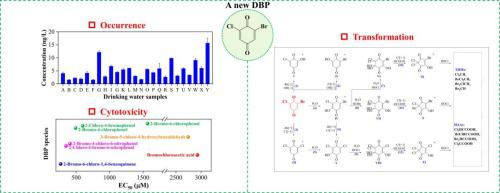
Halobenzoquinones (HBQs) have been reported as an emerging category of disinfection byproducts (DBPs) in drinking water with relatively high toxicity, and the previously reported HBQs include 2,6-dichloro-1,4-benzoquinone, 2,3,6-trichloro-1,4-benzoquinone, 2,6-dichloro-3-methyl-1,4-benzoquinone, 2,6-dibromo-1,4-benzoquinone, 2,6-diiodo-1,4-benzoquinone, 2-chloro-6-iodo-1,4-benzoquinone, and 2-bromo-6-iodo-1,4-benzoquinone. In this study, another HBQ species, 2-bromo-6-chloro-1,4-benzoquinone (2,6-BCBQ), was newly detected and identified in drinking water. The occurrence frequency and levels of 2,6-BCBQ were investigated, and its cytotoxicity was evaluated. Since the formed 2,6-BCBQ was found to be not stable in chlorination, its transformation kinetics and mechanisms in chlorination were further studied. The results reveal that 2,6-BCBQ was generated from Suwannee River humic acid with concentrations in the range of 4.4–47.9 ng/L during chlorination within 120 h, and it was present in all the tap water samples with concentrations ranging from 1.5 to 15.7 ng/L. Among all the tested bromochloro-DBPs, 2,6-BCBQ showed the highest cytotoxicity on the human hepatoma cells. The transformation of 2,6-BCBQ in chlorination followed a pseudo-first-order decay, which was significantly affected by the chlorine dose, pH, and temperature. Seven polar chlorinated and brominated intermediates (including HBQs, halohydroxybenzoquinones, and halohydroxycyclopentenediones) were detected in chlorinated 2,6-BCBQ samples, according to which the transformation pathways of 2,6-BCBQ in chlorination were proposed. Besides, four trihalomethanes and four haloacetic acids were also generated during chlorination of 2,6-BCBQ with molar transformation percentages of 1.6–13.7%.
中文翻译:

新发现的2-溴-6-氯-1,4-苯醌在氯化饮用水中的发生与转化
卤代苯醌(HBQs)是饮用水中新兴的一类消毒副产物(DBPs),毒性较高,此前报道的HBQs包括2,6-二氯-1,4-苯醌、2,3,6-三氯-1,4-苯醌、2,6-二氯-3-甲基-1,4-苯醌、2,6-二溴-1,4-苯醌、2,6-二碘-1,4-苯醌、2-氯-6-iodo-1,4-benzoquinone 和 2-bromo-6-iodo-1,4-benzoquinone。在这项研究中,另一种 HBQ 物种 2-bromo-6-chloro-1,4-benzoquinone (2,6-BCBQ) 在饮用水中被新发现和鉴定。调查了2,6-BCBQ的发生频率和水平,并评价了其细胞毒性。由于发现形成的2,6-BCBQ在氯化中不稳定,因此进一步研究了其在氯化中的转化动力学和机理。结果表明 2, 120 h 内氯化过程中的 ng/L ,它存在于所有自来水样品中,浓度范围为 1.5 至 15.7 纳克/升。在所有测试的溴氯-DBPs中,2,6-BCBQ对人肝癌细胞的细胞毒性最高。氯化过程中 2,6-BCBQ 的转变遵循准一级衰减,这受到氯剂量、pH 和温度的显着影响。在氯化2,6-BCBQ样品中检测到7种极性氯化和溴化中间体(包括HBQ、卤代羟基苯醌和卤代羟基环戊烯二酮),据此提出了2,6-BCBQ在氯化中的转化途径。此外,在 2,6-BCBQ 的氯化过程中还生成了四种三卤甲烷和四种卤乙酸,摩尔转化百分比为 1.6-13.7%。































 京公网安备 11010802027423号
京公网安备 11010802027423号