当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, and characterization of novel pyrazolopyridine and pyridopyrazolopyrimidine derivatives
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2022-05-21 , DOI: 10.1002/jhet.4517 Reda M. Keshk 1
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2022-05-21 , DOI: 10.1002/jhet.4517 Reda M. Keshk 1
Affiliation
|
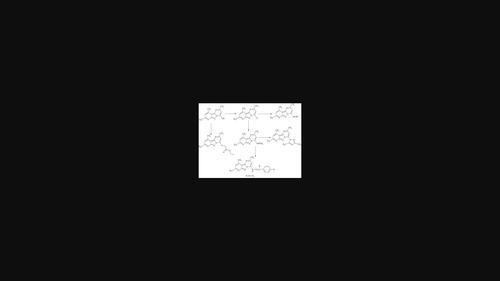
Novel derivatives of pyrazolopyridine and pyridopyrazolopyrimidine were synthesized as these nitrogen heterocyclic compounds have biological and pharmaceutical application. Reaction of 4,6-dimethyl-1H-pyrazolo[3,4-b]pyridin-3-amine 1 with ethyl acetoacetate afforded 3-(4,6-dimethyl-2H-pyrazolo[3,4-b]pyridin-3-ylamino)-but-2-enoic acid ethyl ester 2 and 2,8,10-Trimethylpyrido[2′,3′:3,4]pyrazolo[1,5-a]pyrimidin-4-ol 3. The reaction product depends on the reaction time and catalyst applied. The reaction of ester 2 with POCl3 afforded pyridopyrazolopyrimidine 3 which reacted with ethyl bromoacetate to afford (2,8,10-trimethylpyrido[2′,3′:3,4]pyrazolo[1,5-a]pyrimidin-4-yloxy)acetic acid ethyl ester 4, while the reaction of compound 3 with POCl3 afforded 4-chloro-derivative 5, which reacted with hydrazine and a series of amines (primary and secondary) to yield 6 and 7a–n, respectively. 4-Hydrazino pyridopyrazolopyrimidine 6 reacted with aromatic aldehydes, aromatic ketones, acetylacetone, and ethyl acetoacetate to afford condensation products 8–11. The reaction of ester 2 with ethyl chloroacetate afforded diester 12, which reacted with ethanolamine and hydrazine to afford 13, 14. Pyrazolopyridine 14 reacted with excess acetylacetone to yield pentenone derivative 15. The newly synthesized compound structure was confirmed by elemental analysis and spectral data (IR, NMR and mass spectra).
中文翻译:

新型吡唑并吡啶和吡啶并吡唑并嘧啶衍生物的设计、合成和表征
吡唑并吡啶和吡啶并吡唑并嘧啶的新衍生物合成了这些氮杂环化合物具有生物学和药学应用。4,6-二甲基-1H-吡唑并[3,4-b]吡啶-3-胺1与乙酰乙酸乙酯反应得到3-(4,6-二甲基-2H-吡唑并[3,4-b]吡啶-3 -ylamino)-but-2-enoic acid ethyl ester 2和 2,8,10-Trimethylpyrido[2',3':3,4]pyrazolo[1,5-a]pyrimidin-4-ol 3。反应产物取决于反应时间和所用催化剂。酯2与 POCl 3反应得到吡啶并吡唑并嘧啶3与溴乙酸乙酯反应得到(2,8,10-三甲基吡啶并[2',3':3,4]吡唑并[1,5-a]嘧啶-4-基氧基)乙酸乙酯4 ,化合物3与 POCl 3得到 4-氯衍生物5,其与肼和一系列胺(伯胺和仲胺)反应分别生成6和7a-n。4-肼基吡啶并吡唑并嘧啶6与芳香醛、芳香酮、乙酰丙酮和乙酰乙酸乙酯反应得到缩合产物 8-11 。酯2与氯乙酸乙酯反应得到二酯12,与乙醇胺和肼反应得到13、14。吡唑并吡啶14与过量的乙酰丙酮反应生成戊烯酮衍生物15。通过元素分析和光谱数据(IR、NMR 和质谱)证实了新合成的化合物结构。
更新日期:2022-05-21
中文翻译:

新型吡唑并吡啶和吡啶并吡唑并嘧啶衍生物的设计、合成和表征
吡唑并吡啶和吡啶并吡唑并嘧啶的新衍生物合成了这些氮杂环化合物具有生物学和药学应用。4,6-二甲基-1H-吡唑并[3,4-b]吡啶-3-胺1与乙酰乙酸乙酯反应得到3-(4,6-二甲基-2H-吡唑并[3,4-b]吡啶-3 -ylamino)-but-2-enoic acid ethyl ester 2和 2,8,10-Trimethylpyrido[2',3':3,4]pyrazolo[1,5-a]pyrimidin-4-ol 3。反应产物取决于反应时间和所用催化剂。酯2与 POCl 3反应得到吡啶并吡唑并嘧啶3与溴乙酸乙酯反应得到(2,8,10-三甲基吡啶并[2',3':3,4]吡唑并[1,5-a]嘧啶-4-基氧基)乙酸乙酯4 ,化合物3与 POCl 3得到 4-氯衍生物5,其与肼和一系列胺(伯胺和仲胺)反应分别生成6和7a-n。4-肼基吡啶并吡唑并嘧啶6与芳香醛、芳香酮、乙酰丙酮和乙酰乙酸乙酯反应得到缩合产物 8-11 。酯2与氯乙酸乙酯反应得到二酯12,与乙醇胺和肼反应得到13、14。吡唑并吡啶14与过量的乙酰丙酮反应生成戊烯酮衍生物15。通过元素分析和光谱数据(IR、NMR 和质谱)证实了新合成的化合物结构。































 京公网安备 11010802027423号
京公网安备 11010802027423号