Nuclear Medicine and Biology ( IF 3.6 ) Pub Date : 2022-05-20 , DOI: 10.1016/j.nucmedbio.2022.05.001
Isaac M Jackson 1 , Pablo J Buccino 1 , E Carmen Azevedo 1 , Mackenzie L Carlson 2 , Audrey S Z Luo 1 , Emily M Deal 1 , Mausam Kalita 1 , Samantha T Reyes 1 , Xia Shao 3 , Corinne Beinat 1 , Sydney C Nagy 1 , Aisling M Chaney 1 , David A Anders 1 , Peter J H Scott 4 , Mark Smith 3 , Bin Shen 1 , Michelle L James 5
|
Intro
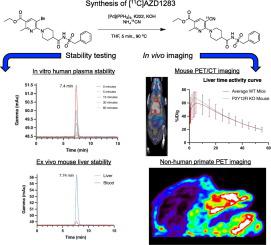
Chronic neuroinflammation and microglial dysfunction are key features of many neurological diseases, including Alzheimer's Disease and multiple sclerosis. While there is unfortunately a dearth of highly selective molecular imaging biomarkers/probes for studying microglia in vivo, P2Y12R has emerged as an attractive candidate PET biomarker being explored for this purpose. Importantly, P2Y12R is selectively expressed on microglia in the CNS and undergoes dynamic changes in expression according to inflammatory context (e.g., toxic versus beneficial/healing states), thus having the potential to reveal functional information about microglia in living subjects. Herein, we identified a high affinity, small molecule P2Y12R antagonist (AZD1283) to radiolabel and assess as a candidate radiotracer through in vitro assays and in vivo position emission tomography (PET) imaging of both wild-type and total knockout mice and a non-human primate.
Methods
First, we evaluated the metabolic stability and passive permeability of non-radioactive AZD1283 in vitro. Next, we radiolabeled [11C]AZD1283 with radioactive precursor [11C]NH4CN and determined stability in formulation and human plasma. Finally, we investigated the in vivo stability and kinetics of [11C]AZD1283 via dynamic PET imaging of naïve wild-type mice, P2Y12R knockout mouse, and a rhesus macaque.
Results
We determined the half-life of AZD1283 in mouse and human liver microsomes to be 37 and > 160 min, respectively, and predicted passive CNS uptake with a small amount of active efflux, using a Caco-2 assay. Our radiolabeling efforts afforded [11C]AZD1283 in an activity of 12.69 ± 10.64 mCi with high chemical and radiochemical purity (>99%) and molar activity of 1142.84 ± 504.73 mCi/μmol (average of n = 3). Of note, we found [11C]AZD1283 to be highly stable in vitro, with >99% intact tracer present after 90 min of incubation in formulation and 60 min of incubation in human serum. PET imaging revealed negligible brain signal in healthy wild-type mice (n = 3) and a P2Y12 knockout mouse (0.55 ± 0.37%ID/g at 5 min post injection). Strikingly, high signal was detected in the liver of all mice within the first 20 min of administration (peak uptake = 58.28 ± 18.75%ID/g at 5 min post injection) and persisted for the remaining duration of the scan. Ex vivo gamma counting of mouse tissues at 60 min post-injection mirrored in vivo data with a mean %ID/g of 0.9% ± 0.40, 0.02% ± 0.01, and 106 ± 29.70% in the blood, brain, and liver, respectively (n = 4). High performance liquid chromatography (HPLC) analysis of murine blood and liver metabolite samples revealed a single radioactive peak (relative area under peak: 100%), representing intact tracer. Finally, PET imaging of a rhesus macaque also revealed negligible CNS uptake/binding in monkey brain (peak uptake = 0.37 Standard Uptake Values (SUV)).
Conclusion
Despite our initial encouraging liver microsome and Caco-2 monolayer data, in addition to the observed high stability of [11C]AZD1283 in formulation and human serum, in vivo brain uptake was negligible and rapid accumulation was observed in the liver of both naïve wildtype and P2Y12R knockout mice. Liver signal appeared to be independent of both metabolism and P2Y12R expression due to the confirmation of intact tracer in this tissue for both wildtype and P2Y12R knockout mice. In Rhesus Macaque, negligible uptake of [11C]AZD1283 brain indicates a lack of potential for translation or its further investigation in vivo. P2Y12R is an extremely promising potential PET biomarker, and the data presented here suggests encouraging metabolic stability for this scaffold; however, the mechanism of liver uptake in mice should be elucidated prior to further analogue development.
中文翻译:

[11C]AZD1283 作为潜在 P2Y12R PET 放射性示踪剂的放射合成和初步临床前评估
介绍
慢性神经炎症和小胶质细胞功能障碍是许多神经系统疾病的关键特征,包括阿尔茨海默病和多发性硬化症。不幸的是,缺乏用于研究体内小胶质细胞的高选择性分子成像生物标志物/探针,P2Y12R 已成为为此目的而探索的有吸引力的候选 PET 生物标志物。重要的是,P2Y12R在中枢神经系统的小胶质细胞上选择性表达,并根据炎症环境(例如,毒性与有益/愈合状态)经历表达的动态变化,因此有可能揭示活体受试者中小胶质细胞的功能信息。在此,我们鉴定了一种高亲和力的小分子 P2Y12R 拮抗剂(AZD1283),通过对野生型和完全敲除小鼠以及非-人类灵长类动物。
方法
首先,我们评估了非放射性AZD1283的体外代谢稳定性和被动渗透性。接下来,我们用放射性前体 [ 11 C ]NH 4 CN放射性标记 [ 11 C ]AZD1283并确定制剂和人血浆中的稳定性。最后,我们通过对初始野生型小鼠、P2Y12R 敲除小鼠和恒河猴进行动态 PET 成像,研究了[ 11 C]AZD1283的体内稳定性和动力学。
结果
我们确定 AZD1283 在小鼠和人肝微粒体中的半衰期分别为 37 分钟和 > 160 分钟,并使用 Caco-2 测定预测了中枢神经系统被动摄取和少量主动外排。我们的放射性标记工作提供了活性为 12.69 ± 10.64 mCi 的[ 11 C]AZD1283,具有高化学和放射化学纯度 (>99%),摩尔活性为 1142.84 ± 504.73 mCi/μmol( n = 3 的平均值)。值得注意的是,我们发现 [ 11 C]AZD1283在体外高度稳定,在制剂中孵育 90 分钟以及在人血清中孵育 60 分钟后,存在 >99% 的完整示踪剂。PET 成像显示健康野生型小鼠 (n = 3) 和 P2Y12 敲除小鼠(注射后 5 分钟时为 0.55 ± 0.37%ID/g)的大脑信号可忽略不计。引人注目的是,在给药的前 20 分钟内,所有小鼠的肝脏中均检测到高信号(注射后 5 分钟的峰值摄取 = 58.28 ± 18.75%ID/g),并在扫描的剩余时间内持续存在。注射后 60 分钟对小鼠组织进行离体伽玛计数,反映了体内数据,血液、大脑和肝脏中的平均 %ID/g 分别为 0.9% ± 0.40、0.02% ± 0.01 和 106 ± 29.70% (n = 4)。对小鼠血液和肝脏代谢物样品的高效液相色谱 (HPLC) 分析显示单个放射性峰(峰下相对面积:100%),代表完整的示踪剂。最后,恒河猴的 PET 成像还显示猴脑中 CNS 摄取/结合可忽略不计(峰值摄取 = 0.37 标准摄取值 (SUV))。
结论
尽管我们最初的肝微粒体和 Caco-2 单层数据令人鼓舞,除了在制剂和人血清中观察到 [ 11 C]AZD1283 的高稳定性外,体内脑摄取可以忽略不计,并且在两种天然野生型的肝脏中观察到快速积累和 P2Y12R 基因敲除小鼠。由于在野生型和 P2Y12R 敲除小鼠的组织中确认了完整的示踪剂,肝脏信号似乎独立于代谢和 P2Y12R 表达。在恒河猴中,[ 11C ]AZD1283 大脑的摄取可以忽略不计,表明缺乏翻译潜力或体内进一步研究的潜力。P2Y12R 是一种非常有前途的潜在 PET 生物标志物,这里提供的数据表明该支架具有良好的代谢稳定性;然而,在进一步开发类似物之前,应阐明小鼠肝脏摄取的机制。



































 京公网安备 11010802027423号
京公网安备 11010802027423号