当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Polycyclic Quinazolinones through C(sp3)−H Functionalization of Inert Alkanes or Visible-Light-Promoted Oxidation Decarboxylation of N-Hydroxyphthalimide Esters
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2022-05-19 , DOI: 10.1002/ejoc.202200523 Wei-Kang Zhang 1 , Jiao-Zhe Li 1 , Can-Can Zhang 1 , Jian Zhang 1 , Yan-Nan Zheng 1 , Yufang Hu 1 , Ting Li 2 , Wen-Ting Wei 3
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2022-05-19 , DOI: 10.1002/ejoc.202200523 Wei-Kang Zhang 1 , Jiao-Zhe Li 1 , Can-Can Zhang 1 , Jian Zhang 1 , Yan-Nan Zheng 1 , Yufang Hu 1 , Ting Li 2 , Wen-Ting Wei 3
Affiliation
|
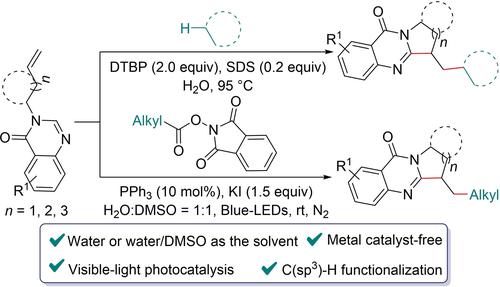
This work describes the cleavage of the C(sp3)−H bond of inert alkanes and the visible-light promoted oxidation decarboxylation of NHP-esters to form alkyl radicals, which then efficiently synthesize valuable polycyclic quinazolinones through radical addition and cyclization. Both methods were carried out in water or water/DMSO media without metal catalysts with good functional group compatibility.
中文翻译:

通过惰性烷烃的 C(sp3)-H 官能化或可见光促进的 N-羟基邻苯二甲酰亚胺酯的氧化脱羧合成多环喹唑啉酮
这项工作描述了惰性烷烃的 C(sp 3 )-H 键的断裂和可见光促进 NHP 酯的氧化脱羧形成烷基自由基,然后通过自由基加成和环化有效合成有价值的多环喹唑啉酮。两种方法均在水或水/DMSO 介质中进行,没有金属催化剂,具有良好的官能团相容性。
更新日期:2022-05-19
中文翻译:

通过惰性烷烃的 C(sp3)-H 官能化或可见光促进的 N-羟基邻苯二甲酰亚胺酯的氧化脱羧合成多环喹唑啉酮
这项工作描述了惰性烷烃的 C(sp 3 )-H 键的断裂和可见光促进 NHP 酯的氧化脱羧形成烷基自由基,然后通过自由基加成和环化有效合成有价值的多环喹唑啉酮。两种方法均在水或水/DMSO 介质中进行,没有金属催化剂,具有良好的官能团相容性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号