European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2022-05-17 , DOI: 10.1016/j.ejmech.2022.114466 Chao Tian 1 , Meng Wang 2 , Xueqi Shi 1 , Xuanzhen Chen 1 , Xiaowei Wang 1 , Zhili Zhang 1 , Junyi Liu 3
|
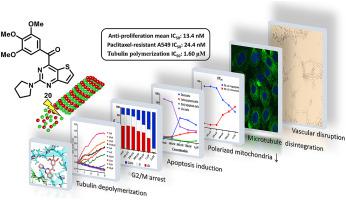
In this study, a series of 2-substituted thieno[3,2-d]pyrimidin-4-yl(3,4,5-trimethoxyphenyl)methanones were designed, synthesized and evaluated as novel anti-tubulin polymerization and vascular disrupting agents. A pyrrolidin-1-yl derivative, compound 20, exhibited strong antiproliferative activities (average IC50 = 13.4 nM) against four cancer cell lines. 20 also showed retained potency toward paclitaxel-resistant A549 cells. 20 could significantly inhibit tubulin polymerization with an IC50 of 1.6 μM. 20 displayed strong induction of G2/M arrest and apoptosis through the mitochondrial pathway. Dose-dependent suppression of the migration of cancer cells and the formation of a vascular network were observed after treatment with 20. The acceptable microsomal stability implied that it is worth conducting further study on the analogues of 20 as novel drug candidates of CBSIs.
中文翻译:

(2-(pyrrolidin-1-yl)thieno[3,2-d]pyrimidin-4-yl)(3,4,5-trimethoxyphenyl)methanone 作为新型强效微管蛋白解聚和血管破坏剂的发现
在本研究中,设计、合成了一系列 2-取代噻吩并[3,2 - d ]嘧啶-4-基(3,4,5-三甲氧基苯基)甲酮作为新型抗微管蛋白聚合和血管破坏剂。pyrrolidin-1-yl 衍生物化合物20 对四种癌细胞系表现出强烈的抗增殖活性(平均 IC 50 = 13.4 nM)。20还显示出对紫杉醇抗性 A549 细胞的保留效力。20可以显着抑制微管蛋白聚合,IC 50为 1.6 μM。20显示通过线粒体途径强烈诱导G2 / M停滞和细胞凋亡。在用20处理后观察到癌细胞迁移的剂量依赖性抑制和血管网络的形成。可接受的微粒体稳定性意味着值得对20的类似物作为 CBSI 的新候选药物进行进一步研究。






























 京公网安备 11010802027423号
京公网安备 11010802027423号