Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2022-05-16 , DOI: 10.1016/j.bioorg.2022.105860 Rongrong Su 1 , Yanyan Diao 1 , Wenjie Sha 1 , Dou Dou 1 , Zhixiao Yu 1 , Limin Leng 1 , Zhenjiang Zhao 1 , Zhuo Chen 1 , Honglin Li 1 , Yufang Xu 1
|
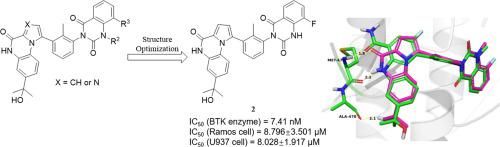
Bruton’s tyrosine kinase (BTK) is a promising target in the treatment of B cell malignancies and autoimmune disorders. Developing selective non-covalent BTK inhibitors is an important strategy to overcome the side effects and drug resistance induced by covalent BTK inhibitors. In this article, we designed and synthesized pyrrolo[1,2-a]quinoxalin-4(5H)-one and imidazo[1,2-a]quinoxalin-4(5H)-one based selective noncovalent BTK inhibitors via scaffold hopping from BMS-986142 and investigated their biological activities. Among the synthesized compounds, pyrrolo[1,2-a]quinoxalin-4(5H)-one derivatives 2 and 4 showed great BTK inhibition potency with IC50 value at 7.41 nM and 11.4 nM, respectively. Besides, they showed equivalent or even better potency in U937 and Ramos cells than BMS-986142. The kinase selectivity profiling study illustrated the excellent selectivity of compound 2 against a panel of 468 kinases. In U937 xenograft models, compound 2 could significantly inhibit tumor growth with TGI = 65.61%. In all, we provided a new scaffold as non-covalent selective BTK inhibitors and the representative compounds exhibited potency both in vitro and in vivo.
中文翻译:

吡咯并[1,2-a]quinoxalin-4(5H)-one 衍生物作为新型非共价布鲁顿酪氨酸激酶 (BTK) 抑制剂的发现
布鲁顿酪氨酸激酶 (BTK) 是治疗 B 细胞恶性肿瘤和自身免疫性疾病的有希望的靶点。开发选择性非共价BTK抑制剂是克服共价BTK抑制剂引起的副作用和耐药性的重要策略。在本文中,我们通过支架设计并合成了基于吡咯并[1,2- a ]quinoxalin-4(5 H )-one 和咪唑并[1,2- a ]quinoxalin-4(5 H )-one 的选择性非共价 BTK 抑制剂。从 BMS-986142 跳跃并研究它们的生物活性。在合成的化合物中,吡咯并[1,2 - a ]quinoxalin-4(5 H )-one衍生物2和4显示出强大的 BTK 抑制效力,IC 50值分别为 7.41 nM 和 11.4 nM。此外,它们在 U937 和 Ramos 细胞中显示出与 BMS-986142 相当甚至更好的效力。激酶选择性分析研究表明化合物2对一组 468 种激酶具有出色的选择性。在 U937 异种移植模型中,化合物2可显着抑制肿瘤生长,TGI = 65.61%。总之,我们提供了一种新的支架作为非共价选择性 BTK 抑制剂,并且代表性化合物在体外和体内均表现出效力。































 京公网安备 11010802027423号
京公网安备 11010802027423号