当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photo-Fenton and oxygen vacancies' synergy for enhancing catalytic activity with S-scheme FeS2/Bi2WO6 heterostructure
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2022-05-17 , DOI: 10.1039/d2cy00610c
Jin Ye 1 , Yuanyuan Zhang 2 , Juan Wang 3 , Shuang Liu 1 , Yuanhang Chang 1 , Xiuping Xu 1 , Chunte Feng 1 , Jian Xu 1, 4 , Li Guo 2 , Jiating Xu 1, 5 , Yujie Fu 1
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2022-05-17 , DOI: 10.1039/d2cy00610c
Jin Ye 1 , Yuanyuan Zhang 2 , Juan Wang 3 , Shuang Liu 1 , Yuanhang Chang 1 , Xiuping Xu 1 , Chunte Feng 1 , Jian Xu 1, 4 , Li Guo 2 , Jiating Xu 1, 5 , Yujie Fu 1
Affiliation
|
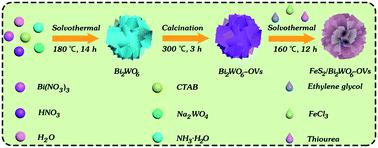
Using calcination and solvothermal methods, a series of FeS2/Bi2WO6 heterojunctions were prepared by coupling FeS2 into the surface oxygen vacancy enriched Bi2WO6. The prepared catalysts were used as photo-Fenton catalysts to degrade tetracycline hydrochloride (TC-HCl) and rhodamine B (RhB). Then, a rational catalytic mechanism where an S-scheme catalyst was constructed between FeS2 and Bi2WO6-OVs was put forward. Furthermore, electron spin resonance (ESR) was employed to confirm that the main active substances in the photo-Fenton degradation process are hydroxyl radicals (˙OH) and superoxide radicals (˙O2−). The enhancement of catalytic activity is mainly due to the synergistic effect between photocatalytic oxidation and Fenton oxidation. And the introduction of oxygen vacancies can not only broaden the light absorption range of the catalyst because of the rise of the valence band but also promote the efficient separation of photogenerated carriers. Besides, the O–O bond of H2O2 could be elongated and weakened due to the presence of surface oxygen vacancies, which can promote the decomposition of H2O2. Meanwhile, the photogenerated electrons in the conduction band (CB) of Bi2WO6-OVs can rapidly transfer to FeS2 near the oxygen vacancies on the catalyst surface, which can achieve a cyclic process from Fe3+ to Fe2+ and boost the degradation efficiency of traditional Fenton catalysis and photocatalysis. The experimental results showed the photo-Fenton rate constants for TC-HCl and RhB over 1%FeS2/Bi2WO6 which were evaluated to be about 0.04430 and 0.02007 min−1 within 50 and 150 min, which was 1.74 and 1.41 times that of the optimum Fenton process, respectively. This strategy showed that the FeS2/Bi2WO6 heterostructure has great advantages in photo-Fenton treatment of multiple organic pollutants due to the synergy of photocatalysis and Fenton catalysis.
中文翻译:

Photo-Fenton 和氧空位的协同作用以增强 S 型 FeS2/Bi2WO6 异质结构的催化活性
采用煅烧和溶剂热法,通过将FeS 2偶联到富含表面氧空位的Bi 2 WO 6中,制备了一系列FeS 2 /Bi 2 WO 6异质结。制备的催化剂用作光芬顿催化剂以降解盐酸四环素(TC-HCl)和罗丹明B(RhB)。然后,提出了在FeS 2和Bi 2 WO 6 -OVs之间构建S型催化剂的合理催化机理。此外,电子自旋共振(ESR)被用来证实光芬顿降解过程中的主要活性物质是羟基自由基(˙OH)和超氧自由基(˙O2 - )。催化活性的提高主要是由于光催化氧化和芬顿氧化的协同作用。并且氧空位的引入不仅可以由于价带的升高而拓宽催化剂的光吸收范围,还可以促进光生载流子的有效分离。此外,由于表面氧空位的存在,H 2 O 2的O-O键会被拉长和弱化,从而促进H 2 O 2的分解。同时,Bi 2 WO 6 -OVs的导带(CB)中的光生电子可以快速转移到FeS 2靠近催化剂表面的氧空位,可以实现从Fe 3+到Fe 2+的循环过程,提高传统芬顿催化和光催化的降解效率。实验结果表明,TC-HCl和RhB在1%FeS 2 /Bi 2 WO 6上的光芬顿速率常数在50和150 min内分别为0.04430和0.02007 min -1,分别为1.74和1.41倍。分别为最优 Fenton 过程。该策略表明 FeS 2 /Bi 2 WO 6由于光催化和芬顿催化的协同作用,异质结构在光芬顿处理多种有机污染物方面具有很大的优势。
更新日期:2022-05-17
中文翻译:

Photo-Fenton 和氧空位的协同作用以增强 S 型 FeS2/Bi2WO6 异质结构的催化活性
采用煅烧和溶剂热法,通过将FeS 2偶联到富含表面氧空位的Bi 2 WO 6中,制备了一系列FeS 2 /Bi 2 WO 6异质结。制备的催化剂用作光芬顿催化剂以降解盐酸四环素(TC-HCl)和罗丹明B(RhB)。然后,提出了在FeS 2和Bi 2 WO 6 -OVs之间构建S型催化剂的合理催化机理。此外,电子自旋共振(ESR)被用来证实光芬顿降解过程中的主要活性物质是羟基自由基(˙OH)和超氧自由基(˙O2 - )。催化活性的提高主要是由于光催化氧化和芬顿氧化的协同作用。并且氧空位的引入不仅可以由于价带的升高而拓宽催化剂的光吸收范围,还可以促进光生载流子的有效分离。此外,由于表面氧空位的存在,H 2 O 2的O-O键会被拉长和弱化,从而促进H 2 O 2的分解。同时,Bi 2 WO 6 -OVs的导带(CB)中的光生电子可以快速转移到FeS 2靠近催化剂表面的氧空位,可以实现从Fe 3+到Fe 2+的循环过程,提高传统芬顿催化和光催化的降解效率。实验结果表明,TC-HCl和RhB在1%FeS 2 /Bi 2 WO 6上的光芬顿速率常数在50和150 min内分别为0.04430和0.02007 min -1,分别为1.74和1.41倍。分别为最优 Fenton 过程。该策略表明 FeS 2 /Bi 2 WO 6由于光催化和芬顿催化的协同作用,异质结构在光芬顿处理多种有机污染物方面具有很大的优势。

































 京公网安备 11010802027423号
京公网安备 11010802027423号