Science China Materials ( IF 6.8 ) Pub Date : 2022-05-13 , DOI: 10.1007/s40843-021-2036-x
Danlei Tang , Lixia Yuan , Yaqi Liao , Wenxuan Jin , Jie Chen , Zexiao Cheng , Xiang Li , Bin He , Zhen Li , Yunhui Huang
|
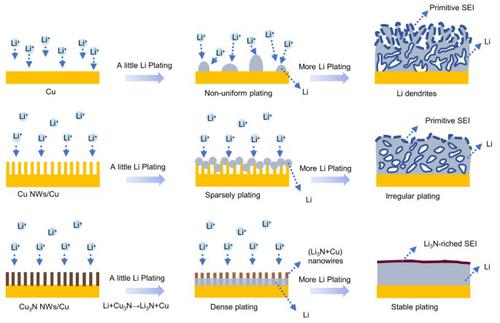
Lithium (Li) metal anodes have the potential to stimulate the development of secondary batteries due to their high theoretical specific capacities and low redox potentials among all possible solid secondary anode compounds. However, the growth of Li dendrites during repeated Li stripping/plating processes leads to low coulombic efficiencies (CEs) and safety hazards, which significantly hinders their practical application. In this work, commercial Cu foil was modified in situ by Cu3N nanowires (Cu3N NWs/Cu) and used as the current collector for a Li anode. In addition to decreasing the true current density of the anode and alleviating the volume change during the cycles, Cu3N reacted with Li during the initial cycle (3Li + Cu3N → Li3N + 3Cu), which enabled the formation of a Li3N-rich solid electrolyte interphase (SEI). This Li3N-rich SEI with a high ionic conductivity not only boosted Li ion transport but also promoted the homogeneous deposition of Li via increased Li nucleation sites. The improvements in both mass transport and deposition dynamics restrained dendrite growth. As a result, the Cu3N NWs/Cu anode had stable Li plating/stripping over 270 cycles with a high average CE of 98.6% at 1 mA cm−2, with Li capacities of 1 mA h cm−2. A long cycling lifespan of 430 cycles was achieved using a full cell with a high-load LiFePO4 cathode (mass loading: 10 mg cm−2) and a Cu3N NWs/Cu-Li anode (N/P = 2.35), demonstrating the effectiveness and practicality of the Cu3N NWs/Cu current collector in stabilizing the Li anode.
中文翻译:

使用Cu3N改性Cu箔作为集流体提高锂金属负极的循环稳定性
锂 (Li) 金属负极在所有可能的固体二次负极化合物中,由于其高理论比容量和低氧化还原电位,有可能刺激二次电池的发展。然而,锂枝晶在重复脱锂/镀锂过程中的生长导致库仑效率(CEs)低和安全隐患,严重阻碍了它们的实际应用。在这项工作中,商业铜箔通过 Cu 3 N 纳米线 (Cu 3 N NWs/Cu)原位改性,并用作锂负极的集流体。除了降低阳极的真实电流密度和缓解循环过程中的体积变化外,Cu 3 N 在初始循环期间与 Li 反应(3Li + Cu3 N → Li 3 N + 3Cu),这使得富Li 3 N的固体电解质中间相(SEI)的形成成为可能。这种具有高离子电导率的富含 Li3N 的 SEI 不仅促进了锂离子的传输,而且通过增加的锂成核位点促进了锂的均匀沉积。质量传输和沉积动力学的改进抑制了枝晶的生长。因此,Cu 3 N NWs/Cu 负极在 270 次循环中具有稳定的锂电镀/剥离,在 1 mA cm -2下具有 98.6% 的高平均 CE,Li 容量为 1 mA h cm -2。使用具有高负载 LiFePO 4阴极(质量负载:10 mg cm-2 ) 和 Cu 3 N NWs/Cu-Li 负极 ( N / P = 2.35),证明了 Cu 3 N NWs/Cu 集流体在稳定锂负极 方面的有效性和实用性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号